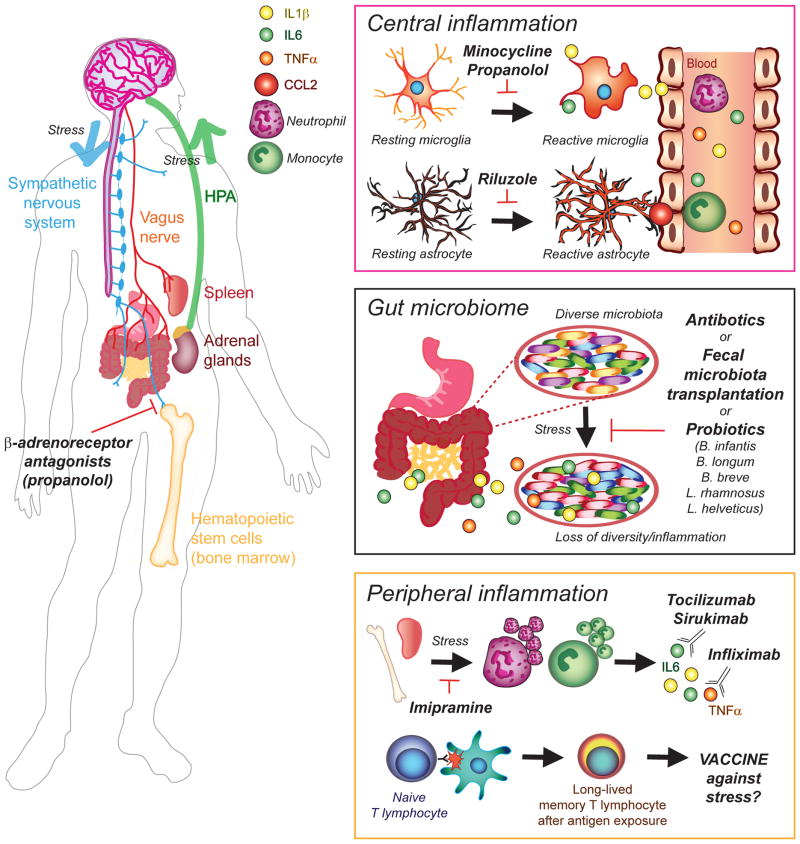
Figure 1. Inflammation-related therapeutic opportunities to treat mood disorders.
Chronic stress activates the sympathetic nervous system promoting release of monocytes and neutrophils from the spleen and bone marrow. Exacerbated stress-induced hematopoietic monocytosis can be blocked by antagonists of β-adrenergic receptors, such as propranolol, reducing peripheral inflammation. Under normal conditions, stimulation of the sympathetic nervous system triggers the hypothalamic-pituitary-adrenal (HPA) axis and glucocorticoid release from the adrenal glands which act as a feedback loop. However, leukocytes of chronically stressed mice are resistant to the anti-inflammatory effects of glucocorticoids maintaining a prolonged inflammatory state and high levels of circulating leukocytes (neutrophils, monocytes) and pro-inflammatory cytokines - Interleukin-1Beta (IL-1β), IL-6, tumor necrosis factor alpha (TNFα) in the blood. These immune signals are then recruited into the brain possibly because of chemokine ligand 2 (CCL2) release by reactive astrocytes. Activation of microglia, the resident immune cells of the brain, is also exacerbated in the context of affective disorders. Central inflammation mediated by these glial cells can be modulated by minocycline or propranolol for microglia, and riluzole for astrocytes, and these compounds have been recently tested in clinical trials as adjunctive therapies to commonly prescribed antidepressants. The gut microbiota can influence the central nervous system and subsequent behavioral responses to stress through activation of the vagus nerve and cytokine release. Loss of microbiota diversity has been reported in stressed mice and depressed patients and associated with increased inflammation and possibly a leaky gut. Thus, administration of antibiotics or probiotics represents novel therapeutic opportunities to treat mood disorders. Fecal microbiotal transplants from patients suffering from major depressive disorder (MDD) recapitulate deleterious behaviors in naive mice raising the possibility to eventually transplant microbiota from healthy donors to treat affective disorders. Recent and ongoing clinical trials are also testing humanized antibodies against inflammatory cytokines – Tocilizumab and Sirukimab for IL-6 and Infliximab for IL-1β - to reduce peripheral inflammation and treat MDD and bipolar disorders. This strategy could be very effective in subsets of patients characterized by pro-inflammatory transcriptional profiles. Finally, preclinical studies suggest that stress could be stored as an immunological memory in T lymphocytes raising the possibility to develop a vaccine against stress-related affective disorders