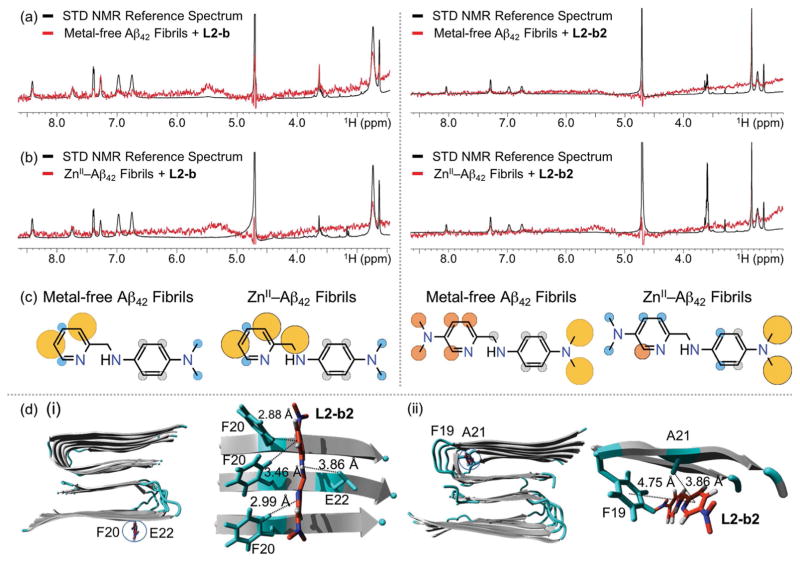
Figure 6.
Interactions of L2-b or L2-b2 with metal-free and ZnII-treated Aβ fibrils. 1H STD NMR spectra of L2-b (left) or L2-b2 (right) in the presence (red) and absence (black) of (a) metal-free or (b) ZnII-added Aβ42 fibrils. Comparison of the STD signal intensities (red) to the STD reference (black) reflects the relative proximity of the corresponding proton from the ligand to Aβ42 fibrils. Conditions: [Aβ42] = 2 μM; [ZnCl2] = 2 μM; [L2-b or L2-b2] = 200 μM; 10 mM Tris-DCl, pD 7.4. (c) Normalized STD intensities mapped on to the structures of L2-b and L2-b2 against metal-free Aβ42 fibrils (left) and ZnII–Aβ42 fibrils (right). Yellow, orange, and blue circles indicate the STD effects of >75 %, 50–75 %, and <50 %, respectively. Gray circles indicate the absence of the STD effect. (d) MD simulations showing interactions of L2-b2 with metal-free Aβ40 fibrils. Two potential binding sites (i and ii) of interaction of L2-b2 with Aβ40 fibrils (PDB 2LMN) after all-atom MD simulations. Right: The zoomed-in view of each binding site with residues showing interaction distances labelled in & with dashed lines. Binding modes (for L2-b) and energies (for both L2-b and L2-b2) are presented in Supporting Information, Figure S8.