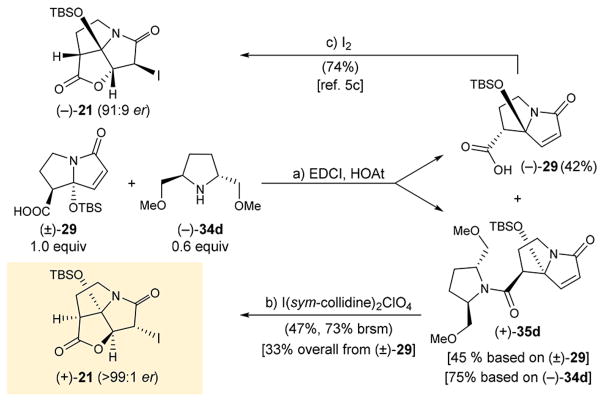
Scheme 2. Enantioselective Synthesis of Iodo Pyrrolizidinone (+)-21a.
aReagents and conditions: (a) (±)-29 (1.0 equiv), EDCI (1.5 equiv), HOAt (1.0 equiv), (−)-34d (0.6 equiv), i-Pr2NEt (3.0 equiv), CH2Cl2, 0°C, 48 h, 75% based on 34d, 45% based on (±)-29; (b) I(sym-collidine)2ClO4 (5.0 equiv), CH2Cl2:MeOH:H2O 1:1:0.05 (v/v/v), 25 °C, 72 h, 47% (73% brsm); (c) I2 (3.0 equiv), NaHCO3 aq:Et2O 1:1 (v/v), 25 °C, 24 h, 74%.