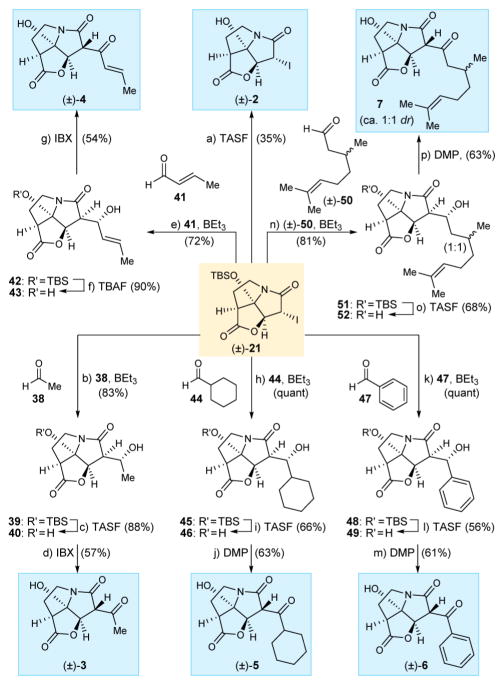
Scheme 4. Synthesis of CJ-16,264 Analogues (±)-2–7a.
aReagents and conditions: (a) TASF (1.8 equiv), THF, 0 °C, 5 min, 35%; (b) (±)-21 (1.0 equiv), 38 (5.0 equiv), BEt3 (1.0 equiv), toluene, −78 °C, 6 h, 83%; (c) TASF (1.5 equiv), THF, 0 °C, 5 min, 88%; (d) IBX (5.0 equiv), EtOAc, 70 °C, 6 h, 57%; (e) (±)-21 (1.0 equiv), 41 (3.0 equiv), BEt3 (1.1 equiv), toluene, −78 °C, 6 h, 72%; (f) TBAF (1.0 equiv), THF, 0 °C, 5 min, 90%; (g) IBX (6.0 equiv), EtOAc, 70 °C, 6 h, 54%; (h) (±)-21 (1.0 equiv), 44 (5.0 equiv), BEt3 (1.0 equiv), toluene, −78 °C, 6 h, quant; (i) TASF (1.5 equiv), THF, 0 °C, 5 min, 66%; (j) DMP (1.5 equiv), CH2Cl2, 0 °C, 1.5 h, 63%; (k) (±)-21 (1.0 equiv), 47 (3.0 equiv), BEt3 (1.1 equiv), toluene, −78 °C, 6 h, quant; (l) TASF (1.5 equiv), THF, 0 °C, 5 min, 56%; (m) DMP (1.5 equiv), CH2Cl2, 0 °C, 1.5 h, 61%; (n) (±)-21 (1.0 equiv), 50 (3.0 equiv), BEt3 (1.0 equiv), toluene, −78 °C, 6 h, 81% (1:1 dr); (o) TASF (1.5 equiv), THF, 0 °C, 5 min, 68% (1:1 dr); (p) DMP (1.5 equiv), CH2Cl2, 0 °C, 1.5 h, 63% (1:1 dr); TASF = tris(dimethylamino)sulfonium difluorotrimethylsilicate.