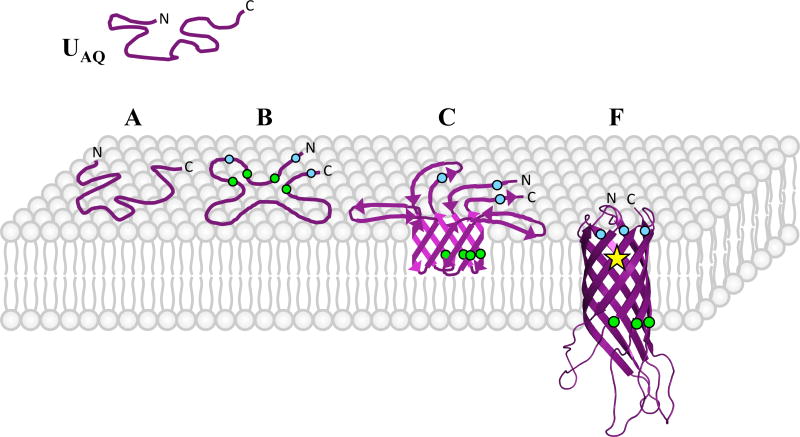
Figure 7.
Illustration of the proposed on-pathway intermediate states in the folding mechanism for the OmpA β-barrel. The attributes of each species are summarized in Table S10 of the Supporting Information. UAQ is a folding competent, non-membrane-associated unfolded state. Upon membrane association, UAQ converts to species A, which contains no regular structure. A then converts to species B, which has no regular secondary structure but has a loose arrangement of β-hairpins in a “clover-like” shape, oriented parallel to the membrane surface. Site-directed quenching studies have revealed that residues on the translocating (trans) portions of adjacent strands (green circles) are in closer proximity in this state than residues on the periplasmic (cis) portions of strands (blue circles).55 B converts to species C, which is partially inserted and contains a higher β-sheet content than the native state. For this reason, the portions of the strands remaining on the surface as well as the membrane-embedded extracellular loops are proposed to be engaged in H-bonded β-strand conformations in addition to the membrane-inserted portion of the β-barrel. The closer-proximity trans residues are again indicated by green circles and the cis residues as blue circles. Full insertion of intermediate C leads to formation of the native β-barrel structure, F (OmpA171 PDB id 1BXW61). This state exhibits a shifted SDS-PAGE migration and an aromatic exciton signal in the CD spectrum at 230 nm. The interacting residues responsible for this signal are unknown, but the interaction only occurs in the native state and is depicted by a yellow star. The trans and cis residues used in site-directed quenching experiments are again indicated by green and blue circles, respectively.