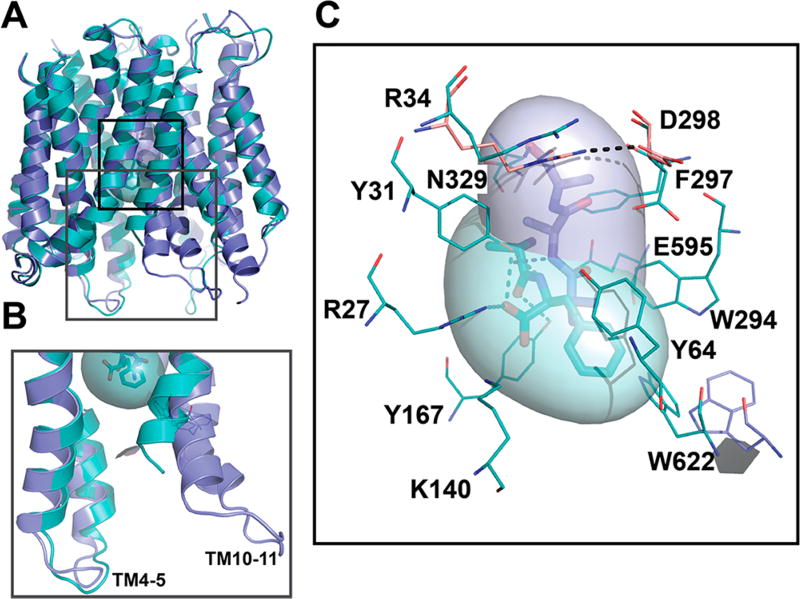
Figure 1.
hPepT1 models in the inward-open conformations. (A) hPepT1 inward-open models bound to the dipeptide Ala-Phe (Model 4; cyan) the tripeptide Ala-Ala-Ala (Model 5; purple) are shown in cartoon. (B) Two conformations of the internal gate, constituted by the hairpin loops TM4-5 and TM10-11 are shown in cartoon for Models 4 (cyan) and 5 (purple). (C) The binding site residues of the inward-open conformation of hPepT1 based on the Ala-Phe bound template are shown in cyan lines. An alternative orientation of W622 from the Ala-Ala-Ala bound structure is shown in purple lines. The closed gate, constituted by a salt bridge between R34 and D298 is shown in pink lines, where the coordinates are derived from our model based on the unbound inward-open conformation (Table 1; Model 2). The coordinates of the peptide substrates Ala-Phe and Ala-Ala-Ala, shown in sticks and surface representations, are derived from the corresponding template structures. The hydrogen bonds established between the ligands and hPepT1 binding site are represented in dashed lines.