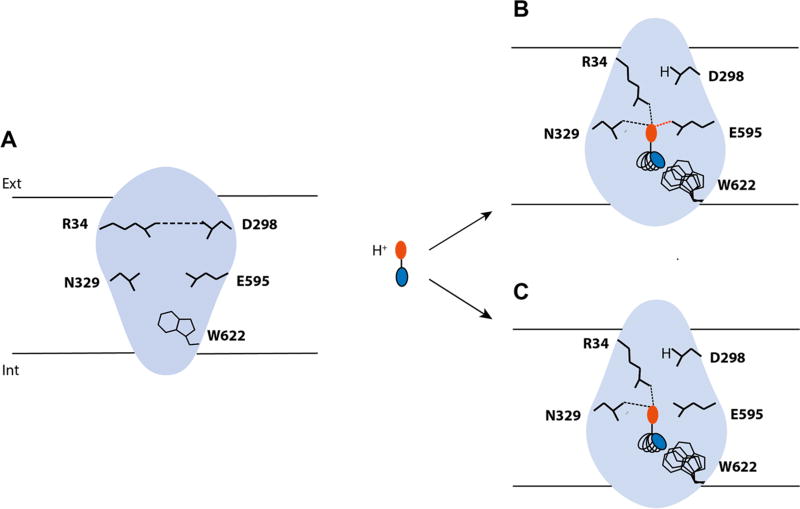
Figure 5.
hPepT1 binding site plasticity and ligand promiscuity. hPepT1 binding site is represented in blue and the key residues interacting with the ligands in black lines. (A) When no ligand is bound to hPepT1, the salt bridge between R34 and D298 (i.e., the external gate) is formed. (B) When a peptide binds, the N-terminus of the peptide, represented in red, disrupts the hydrogen bond to interact with R34 and other key residues such as E595 or N329. Additionally, D298 binds the cotransported proton. (C) Conversely, when an inhibitor binds to hPepT1, key interactions essential for transport are lacking, such as the hydrogen bond between the ligand with E595. The flexibility of the internal side of the binding site permits to accommodate the C-terminal side of the peptide or peptide-like substrates and drugs and inhibitors (represented in blue) of various sizes and shapes.