Abstract
Individuals with psychosis often show high levels of intrinsic, or nonspecific, neural activity, but attenuated stimulus-specific activity. Clementz et al. (2016) proposed that one subgroup of psychosis cases has accentuated intrinsic activity (Biotype-2’s) and a different subgroup (Biotype-1’s) has diminished intrinsic activity, with both groups exhibiting varying degrees of cognitive deficits. This model was studied by assessing neural activity in psychosis probands (N=105) during baseline and a 5sec period in preparation for a pro-/anti-saccade task. Steady-state stimuli allowed real-time assessment of modulation of visuocortical investment to different target locations.
Psychosis probands as a whole showed poor antisaccade performance. As expected, Biotype-1 showed diminished intrinsic neural activity and the worst behavior, and Biotype-2 showed accentuated intrinsic activity and less deviant behavior. Both of these groups also exhibited less dynamic oscillatory phase synchrony. Biotype-3 showed no neurophysiological differences from healthy individuals, despite a history of psychosis. Interestingly, all psychosis subgroups showed normal (i.e., not different from healthy) preparatory modulation of visuocortical investment as a function of cognitive demands, despite varying levels of task performance. Similar analyses conducted subgrouping cases by psychotic symptomatology revealed fewer and less consistent differences, including no intrinsic activity differences between any clinical subgroup and healthy individuals. This study illustrates that (i) differences in intrinsic neural activity may be a fundamental characteristic of psychosis and need to be evaluated separately from stimulus-specific responses, and (ii) grouping patients based on multidimensional classification using neurobiological data may have advantages for resolving heterogeneity and clarifying illness mechanisms relative to traditional psychiatric diagnoses.
Keywords: psychosis, EEG, biomarkers, schizophrenia, bipolar disorder, steady-state
1. Introduction
Intrinsic, or nonspecific, neural activity is not directly linked to stimulus processing, though it can be correlated with sensory cortical responses (e.g., Clementz et al., 2008; Spencer et al., 2004; Wang et al., 2010). Individuals with psychosis often show high levels of intrinsic neural activity (Clementz et al., 1994; Clementz and Blumenfeld, 2001; Krishnan et al., 2005; Rolls et al., 2008; Winterer et al., 2006, 2004, 2000; Winterer and Weinberger, 2004). For instance, in response to periodically flickering visual stimuli, Ethridge et al. (2011) observed augmented baseline and stimulus-related activity among psychosis patients compared to healthy persons. Levels of stimulus-specific activity (brain activity to the stimulus minus baseline activity), however, were diminished in psychosis.
Clementz et al. (2016) proposed, given similarity across a range of brain-based biomarkers, that a distinct subgroup of psychosis cases will have high levels of intrinsic activity and excessive reactivity to sensory stimuli (Biotype-2) while a different subgroup will have diminished intrinsic activity and low levels of sensory reactivity (Biotype-1). Hudgens-Haney et al. (2017) found that Biotype-2 and Biotype-1 cases indeed exhibited these diametrically opposed differences in baseline and stimulus-related activity in response to centrally located flickering visual stimuli. It has been theorized that either abnormally high or low intrinsic activity may lead to low signal-to-noise ratios, reducing the ability to parse stimulus relevance and accurately code perceptual events (Rolls et al., 2008). In the case of Hudgens-Haney et al. (2017), either high or low levels of intrinsic activity in psychosis were associated with decreased cognitive control, suggesting that modes of facilitating context-appropriate behavior through modulation of sensory systems may differ between distinct psychosis subgroups. Under this hypothesis, networks characterized by hyperexcitation would likely show less extensive deficits than those characterized by hypoexcitation (Rolls et al., 2008), consistent with less deviant behavioral performance in Biotype-2 than Biotype-1 (Hudgens-Haney et al., 2017).
Individuals with psychosis have difficulty inhibiting context-inappropriate behavioral responses, illustrated by high error rates on antisaccade tasks (McDowell et al., 1999; Reilly et al., 2014). Unlike prosaccade tasks, where a glance is made towards a peripheral cue, antisaccade tasks require inhibiting a glance to a peripheral cue and instead looking to that cue’s mirror image location (Hallett, 1978). Neurophysiology studies indicate that suppression of reflexive saccades during antisaccade tasks requires preparatory modulation of neural activity in visual cortex (Munoz and Everling, 2004), theoretically generated via top-down control by prefrontal cortex (Johnston and Everling, 2006). Healthy persons and those with psychosis have been found to increase visuocortical neural investment to central stimuli (i.e., activity related to these stimuli) prior to anti- versus pro-saccade responses (Hudgens-Haney et al., 2017). Although the complimentary process, decreased visuocortical investment to peripheral stimuli prior to anti- versus pro-saccades, has been found in healthy persons (Clementz et al., 2010), this has not been investigated in individuals with psychosis. This is particularly relevant as Clementz et al. (2008) found the vSSR in individuals with SZ to be deviant specifically in relation to targets, while activity related to non-targets was unaffected.
The present study used peripherally-located oscillating steady-state visual stimuli to assess modulation of neural activity during preparation for pro- or anti-saccades. The visual steady state response (vSSR) develops in primary visual cortex in relation to stimuli flickering at a specific rate (Clementz et al., 2008; Di Russo et al., 2006; Pastor et al., 2003). The resultant oscillations resonate at the stimulus flicker rate and vary in amplitude with the task relevance of the stimulus (Wang et al., 2007). Given findings from Hudgens-Haney et al. (2017), we expected that (i) both behavioral performance and brain activity measures would differentiate psychosis Biotypes (Clementz et al., 2016); (ii) Biotype-1 would have the worst antisaccade performance and diminished intrinsic neural activity, Biotype-2 would have less deviant antisaccade performance but accentuated intrinsic activity, Biotype-3 would be most similar to healthy subjects on both performance and brain responses; and (iii) neural synchrony deviations would be found for Bioype-1 and Biotype-2, but not for Biotype-3.
2. Methods and Materials
2.1. Participants
As a part of a multisite data collection project, the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP), subjects were recruited, interviewed, and tested for the present study at the University of Illinois-Chicago site. Clinically stable psychosis probands (N = 105) and healthy comparison subjects (HC; N =50) were recruited via community advertisements, linked community facilities and programs, and family support groups (see Table 1 and Supplemental Table 1 and 2 for clinical and demographic information). Clinical diagnosis was determined through administration of the Structured Clinical Interview for DSM-IV Diagnosis (First et al., 1997) and consensus diagnostic review; see Tamminga et al. (2013) for clinical evaluation details. HC had no personal history of psychotic, bipolar, or recurrent major depressive disorder, or a family history of schizophrenia-bipolar spectrum disorders in first or second degree relatives (Tamminga et al., 2013). All subjects provided written consent before participating, and the study was approved by the University of Illinois-Chicago Institutional Review Board.
Table 1.
Demographic and Clinical Characteristics of Healthy Comparison Subjects and Probands as a Function of Biotype
| Percent Female | Healthy (N=50) | Biotype-1 (N=20) | Biotype-2 (N=30) | Biotype-3 (N=55) | Significance Test | ||||
|---|---|---|---|---|---|---|---|---|---|
| 56 | 70 | 53 | 44 | χ2(3)=4.44, p=.22 | |||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| Age | 37.9 | 12.2 | 31.8 | 13.9 | 32.3 | 12.2 | 35.5 | 13.4 | F(3,151)=1.75, p=.16 |
|
| |||||||||
| Trials accepted | |||||||||
| Prosaccades | 47.6 | 4.4 | 48.2 | 2.7 | 48.1 | 2.8 | 47.3 | 3.5 | F(3,151)=0.43, p=.73 |
| Antisaccades | 48.0 | 3.4 | 48.2 | 2.5 | 48.1 | 2.7 | 47.1 | 5.5 | F(3,151)=0.74, p=.53 |
|
| |||||||||
| Percent Correct | |||||||||
| Prosaccades | 97.2 | 3.2 | 94.1 | 6.0 | 94.6 | 6.1 | 95.8 | 4.5 | F(3,151)=3.0, p=.03 |
| Antisaccades | 90.7 | 6.3 | 77.4 | 12.2 | 86.1 | 11.7 | 83.6 | 11.1 | F(3,151)=9.30, p=.00001 |
|
| |||||||||
| Response Latency (ms)a | |||||||||
| Correct Prosaccades | 376.7 | 55.0 | 379.8 | 85.4 | 365.3 | 76.9 | 366.3 | 70.8 | F(3,151)=0.37, p=.78 |
| Correct Antisaccades | 415.1 | 73.5 | 455.7 | 103.5 | 437.4 | 91.2 | 428.6 | 84.0 | F(3,151)=1.20, p=.31 |
| Incorrect Antisaccades | 352.2 | 102.9 | 331.3 | 75.0 | 350.4 | 132.1 | 361.2 | 89.0 | F(3,145)=0.42, p=.74 |
|
| |||||||||
| GAFb | 84.56 | 5.4 | 43.6 | 8.2 | 49.5 | 11.3 | 53.7 | 13.7 | F(2,102)=5.26, p=.007 |
|
| |||||||||
| BACS | −0.05 | 1.2 | −2.6 | 0.7 | −1.8 | 1.0 | −0.3 | 0.8 | F(2,99)=65.3, p=8E-19 |
|
| |||||||||
| Birchwood Social Functioning Scale | 163.0 | 18.9 | 126.6 | 23.8 | 130.1 | 18.8 | 133.7 | 23.2 | F(2,77)=0.59, p=.56 |
|
| |||||||||
| PANSS-positive | – | – | 19.0 | 7.8 | 15.6 | 4.8 | 15.7 | 5.0 | F(2,101)=2.84, p=.06 |
|
| |||||||||
| PANSS-negative | – | – | 20.3 | 7.8 | 15.3 | 5.3 | 15.7 | 6.0 | F(2,101)=4.53, p=.01 |
|
| |||||||||
| PANSS-general | – | – | 38.3 | 10.4 | 33.4 | 7.8 | 34.3 | 8.0 | F(2,101)=2.19, p=.12 |
|
| |||||||||
| MADRS | – | – | 14.8 | 9.4 | 10.4 | 8.6 | 10.1 | 8.5 | F(2,101)=2.25, p=.11 |
|
| |||||||||
| Young Mania Scale | – | – | 7.3 | 6.5 | 5.9 | 5.2 | 6.3 | 6.6 | F(2,102)=0.28, p=.75 |
|
| |||||||||
| Medication Class | |||||||||
| Antipsychotic first generation | 0.0% | 15.0% | 10.0% | 5.5% | χ2(2)=1.82, p=.40 | ||||
| Antipsychotic second generation | 0.0% | 75.0% | 73.3% | 61.8% | χ2(2)=1.79, p=.41 | ||||
| Mood stabilizer | 0.0% | 50.0% | 46.7% | 47.3% | χ2(2)=0.06, p=.97 | ||||
| Lithium | 0.0% | 5.0% | 20.0% | 16.4% | χ2(2)=2.20, p=.33 | ||||
| Antidepressant | 2.0% | 45.0% | 40.0% | 43.6% | χ2(2)=0.15, p=.93 | ||||
| Sedative/anxiolytic | 2.0% | 30.0% | 23.3% | 25.5% | χ2(2)=0.28, p=.87 | ||||
| Stimulant | 0.0% | 5.0% | 3.3% | 14.5% | χ2(2)=3.42, p=.18 | ||||
| Anticholinergic | 0.0% | 10.0% | 6.7% | 7.3% | χ2(2)=0.21, p=.90 | ||||
GAF, Global Assessment of Functioning; PANSS, Positive and Negative Symptom Scale; MADRS, Montgomery–Åsberg Depression Rating Scale; BACS, Brief Assessment of Cognition in Schizophrenia.
Only 40% of participants made any incorrect responses during prosaccade trials.
Healthy subjects were excluded from comparisons of clinical characteristics and medication status.
Clementz et al. (2016) used biomarker data to classify psychosis cases into Biotypes. Biotype-1 was most reminiscent of poor-outcome, chronic, and neurobiologically impaired psychosis cases; Biotype-2 was characterized by hyper-responsiveness to sensorimotor events, while Biotype-3 was closer to HC than to the other Biotype subgroups across biomarkers (despite presence of psychosis), although they still had modest deviations on multiple biomarkers. All participants in the current study were included in Clementz et al. (2016), and so their current Biotype membership (Biotype-1, N=20; Biotype-2, N=30; Biotype-3, N=55) is known. The data used for developing Biotypes was independent, however, from tasks and measurements obtained for the present study (see Supplemental Materials). The current data were collected as part of a series of complimentary paradigms on the same sample as reported in Hudgens-Haney et al. (2017). Sample size differences are due to EEG data quality. The recording and preprocessing of EEG data were identical to that in previous work (Hudgens-Haney et al., 2017; also see Supplemental Materials), except that here the data were kept at the original sampling rate of 1000 Hz.
2.2. Stimuli
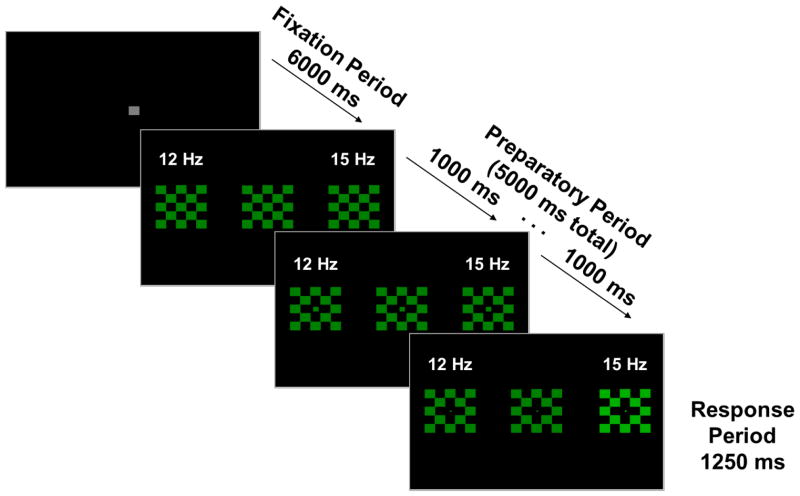
Stimuli were presented on a 19-inch flat-surface computer monitor with a refresh rate of 60Hz. Stimuli consisted of three 5×5cm checkerboards (alternating colored and black squares, each 1deg square) located at central fixation and 8 deg to the left and right of central fixation (Figure 1). Checkerboards were either green (during the prosaccade block) or red (during the antisaccade block). Both colors had equal luminance (5 cd/m2). The peripheral checkerboards were luminance modulated (100% modulation depth) at two flickering frequencies (12Hz in the left visual field, 15Hz in the right visual field), with the central checkerboard not flickering. Blocks consisted of 50 trials of either pro- or anti-saccades, for a total of 100 trials.
Figure 1.
Example Prosaccade Trial. During the antisaccade block, stimuli would be red rather than green. Peripheral checkerboards flickered at 12 Hz (left) and 15 Hz (right), with the central checkerboard not flickering. The frequency labels (white) are for visualization and were not present during the task. At each quarter of the Fixation Period (1500 ms), the central fixation square decreased in size by ¼ in order to provide a “countdown” to the next trial. Similarly, at 1000 ms intervals during the Preparatory Period, the central square of the central checkerboard decreased in size by ¼ in order to provide the participants information about the impending response requirement. After the 5 sec Preparatory Period, one of the peripheral checkerboards (pseudo-randomly determined) increased in luminance for 1.25 sec (the saccadic response period), while both peripheral checkerboards continued flickering. On prosaccade trials, participants were to look as quickly and accurately as possible toward this target; on antisaccade trials, participants were instructed to look as quickly and accurately as possible to the mirror image location (opposite peripheral checkerboard). All analyses are time-locked to the onset of the Preparatory Period. The Response Period was used solely to assess behavior; no brain data from this period are included in the analyses.
Each trial was preceded by an inter-trial interval of 6sec, in which a gray central fixation square (1 deg square) was presented. At each quarter of this Fixation Period (1.5sec), the central square decreased in size by ¼ in order to give a “countdown” to the next trial. Trials began with the peripheral checkerboards flickering for 5 sec. Participants were instructed to maintain fixation on the central checkerboard throughout this Preparatory Period. Similar to the Fixation Period, at 1sec intervals during the Preparatory Period the center square of the central checkerboard decreased in size by ¼ in order to give the participants information about the impending response requirement. After the 5sec Preparatory Period, one of the peripheral checkerboards (pseudo-randomly determined) increased in luminance to 20 cd/m2 for 1.25sec (the saccadic Response Period), while both peripheral checkerboards continued flickering. On prosaccade trials, participants were to look as quickly and accurately as possible toward this target; on antisaccade trials, participants were instructed to look as quickly and accurately as possible to the mirror image location (opposite checkerboard). All analyses were time-locked to the onset of the Preparatory Period, and descriptions below (e.g., pre or post stimulus onset) reflect this. As the primary interest of this study was preparatory modulation, analyses do not include any brain data from the Response Period.
2.3. Data Analyses
Two approaches were used to quantify brain activity. First, evoked potentials were used to evaluate the amplitude and spatial distribution of brain activity at the onset of visual stimulation (at the beginning of the Preparatory Period, before stabilization of the steady-state response). Second, as in Clementz et al. (2010), spectral measures were used to assess the power, phase stability, and spatial distribution of the vSSR over time before and during the Preparatory Period (−500ms to 5000ms in relation to stimuli onset). For all analyses, Greenhouse-Geisser correction was used when sphericity was violated (Mauchly, 1940). All analyses were replicated as a function of subgrouping psychosis cases by clinical symptomatology (see Supplemental Results).
2.3.1. Visual Evoked Potentials
The voltage data were used to test for differences in brain activations, at the beginning of visual processing at Preparatory Period onset, between the pro- and anti-saccade conditions and between groups (Figure 2; Clementz et al., 2010, 2007; McDowell et al., 2005), using methods identical to previous work (Hudgens-Haney et al., 2017; also see Supplemental Materials).
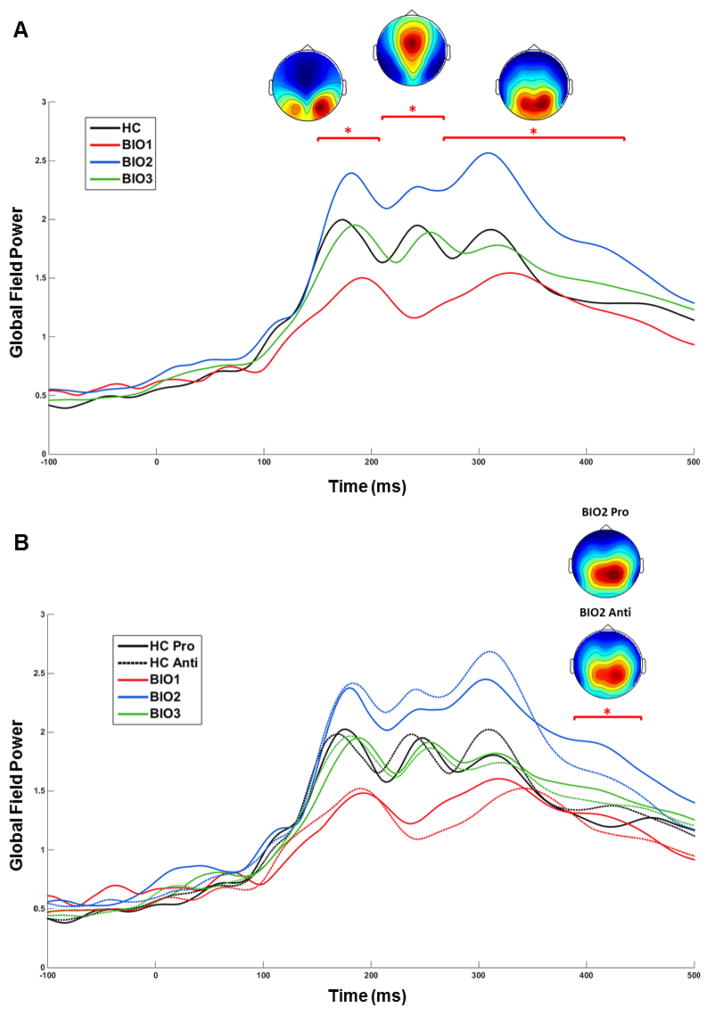
Figure 2.
Evoked Potentials to the Onset of the Preparatory Period. For each participant, EEG data from 250ms before to 500 ms after the onset of the Preparatory Period were averaged across trials and baseline-subtracted using the average of −250:0 ms. Global field power (GFP; the root mean square of voltage over all sensors at each time point) was calculated for each participant and task. Waveforms were averaged into 10 ms bins and analyzed using a 2-way ANOVA (Group × Saccade Type) at each time bin. To control for increased family-wise error rate due to multiple comparisons, a clustering method (Forman et al., 1995) was used to take account of the non-independence of data from adjacent time bins (for examples, see Hamm et al. 2012; Hudgens-Haney et al., 2013; and Monte Carlo simulations, Cox, 1996). Time bin “clusters” were considered significant at overall family-wise α < .01 if at least three adjacent time bins were significant at p < .05. Surviving time bin clusters were subjected to planned post hoc t-tests. (A) The colored top-down topographies are grand average voltage maps (μV) from the three time bin clusters at which a significant Main Effect of Group was found. (B) The topographies are voltage maps for Biotype-2 from pro- and anti-saccade trials during the time bin cluster at which a significant Group × Task Interaction was found. *p<.05
2.4.2. Spectral measures
Similar to previous work using paradigms comparable to those used in the present study (Clementz et al., 2010; Hudgens-Haney et al., 2017), the vSSR was quantified using inter-trial phase coherence (ITC; increased across-trial phase similarity of the EEG signals in relation to frequency of visual flickering stimuli) and single-trial power (STP). ITC and STP of the steady-state response across time were estimated by complex demodulation at the flickering rates (12 and 15 Hz) using published methods (Clementz et al., 2010; Moratti et al., 2007; also see Supplemental Materials). Spectral values for the two driving frequencies were initially analyzed individually but were later summed as frequency did not systematically interact with group, task, or time. ITC values were averaged over 9 posterior cortex sensors capturing peak vSSR activity (Supplemental Figure 1; Clementz et al., 2010, 2008; Ethridge et al., 2011; Wang et al., 2007), averaged from −500:0ms pre-stimulus and over each of the five 1sec Preparatory Period countdowns, and analyzed using mixed effects 3-way MANOVAs (Group × Saccade Type × Time Bin) with post hoc Tukey honestly significant difference tests.
To most accurately and comprehensively capture the shared variance in spatial topographies of STP across time, spatial PCA (Carroll et al., 2008; Clementz and Blumenfeld, 2001) was implemented using the ERP PCA Toolkit (Dien, 2012) in Matlab (Mathworks, Matick, MA). For each group and task individually, power values from −500:5000ms were standardized across sensors before averaging across subjects. Scree tests for each identified one component. The spatial distribution and amount of variance accounted for were nearly identical (all correlations greater than r = .975), so the same procedure was repeated on a single grand average time course, resulting in a component accounting for 48.6% of variance (see Supplemental Figure 2). Component weights were multiplied by each subject’s data, summed across sensors, and divided by the absolute square sum of the component weights, reducing STP waveforms from 64 (one for each sensor) to 1 for each participant. STP averaged over the entire 5500ms time course provided a measure of intrinsic activity and were subjected to a 2-way ANOVA (Group × Saccade Type) with post hoc Tukey honestly significant difference tests. Averaged values for each of the five 1sec Preparatory Period countdowns were baseline-subtracted and analyzed using a mixed effects 3-way MANOVAs (Group × Saccade Type × Time Bin) with post hoc Tukey honestly significant difference tests. Induced power was also examined as an alternative measure of intrinsic neural activity, with results similar to those of STP (see Supplemental Materials).
3. Results
3.1. Behavioral Data
As expected, correct antisaccades had longer latencies than correct prosaccades, F(1,151)=134.93, p=1.09E-22 (Table 1), which is thought to represent additional computational requirements for task performance (McDowell et al., 1999). A Group × Task interaction for latency of correct responses, F(3,151)=2.88, p=.04, was driven by HC showing faster antisaccade responses than Biotype-1.
Error rate was greater for anti- than pro-saccades, F(1,151)=154.57, p=7.0E-25. Group differences, F(3,151)=9.78, p=.000006, and Group × Task interactions, F(3,151)=5.89, p=.001, were driven by HC making fewer antisaccade errors than all psychosis groups, as well as Biotype-1making more antisaccade errors than all other groups. This is consistent with the findings of Clementz et al. (2016) using a different antisaccade testing paradigm (Reilly et al., 2014), which found Biotype-1 to be most prototypical of the chronic, poor-outcome cases often considered to capture the essence of schizophrenia (Keshavan et al., 2013, 2011).
3.2. Evoked Potentials to Checkerboard Onset
At the onset of the checkerboard stimuli, there were four clearly identifiable peaks in global field power (GFP) prior to the onset of the vSSR (Figure 2). The latencies of these peaks (135ms, 175ms, 245ms, and 320ms) are consistent with studies using similar stimuli (Clementz et al., 2010; Ethridge et al., 2011; Hudgens-Haney et al., 2017). Group differences in GFP were found from 150–450ms, F(3,151)=4.08, p=.014 (Figure 2A), with Biotype-2 showing greater GFP than all other groups. From 150–330ms, GFP was lower in Biotype-1 than all other groups. A Group × Task interaction effect was found at 400–430ms, F(3,151)=3.88, p=.011, which was driven by Biotype-2 showing greater GFP during pro- than anti-saccade trials (Figure 2B). These opposing deviations in visuocortical responsiveness (hypoactivity in Biotype-1 and hyperactivity in Biotype-2) are consistent with results from auditory tasks (Ethridge et al., 2015; Hamm et al., 2014).
3.3. Single-Trial Oscillatory Power
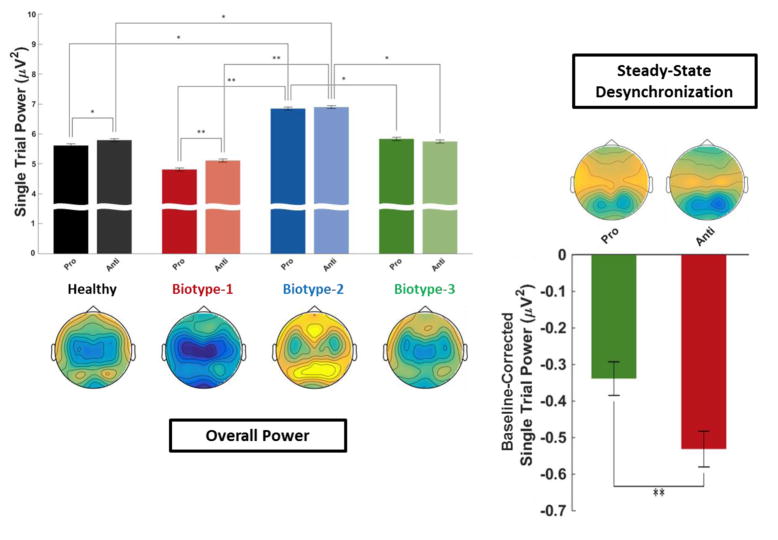
Intrinsic neural activity (STP averaged from −500ms to 5000ms in relation to onset of the Preparatory Period) showed a main effect of Task, F(1,151)=5.07, p=.026, being greater during anti- than pro-saccade blocks (Figure 3). This presumably reflects changed activity levels in the basic saccade circuitry and/or activity in newly recruited neural regions (McDowell et al., 2008; Sweeney et al., 1996). There was a main effect of Group, F(3,151)=3.94, p=.01, with Biotype-2 greater than all other groups, as proposed by Clementz et al. (2016). There was also a Group × Task interaction, F(3,151)=3.12, p=.028, driven by only HC and Biotype-1 showing greater intrinsic activity during anti- than pro-saccade blocks.
Figure 3.
Single-Trial Oscillatory Power. Top-down topographies of oscillatory power averaged over the steady-state driving frequencies (12 & 15Hz) for intrinsic activity (power averaged from −500ms to 5000ms in relation to stimulus onset; left) and stimulus-specific (i.e., baseline-subtracted) power (right). Bar plots represent PCA-weighted power (see Supplemental Figure 2). Error bars indicate standard error of the mean. For intrinsic activity (PCA-weighted power averaged from −500:5000 ms) on the left, a 2-way ANOVA (Group × Saccade Type) was calculated, with post hoc Tukey honestly significant difference tests on the estimated marginal means. For task-specific desynchronization (i.e., baseline-corrected PCA-weighted power) on the right, a mixed effects 3-way MANOVA (Group × Saccade Type × Time Bin) was calculated, with post hoc Tukey honestly significant difference tests on the estimated marginal means. *p<.05, **p<.01
Stimulus-specific (i.e., baseline-subtracted) power showed a main effect of Task, F(1,151)=8.06, p=.005, with greater desynchronization observed during anti- than pro-saccade trials. This effect is expected based on nonhuman primate neurophysiology studies indicating that inhibition of reflexive saccades during antisaccade tasks requires suppression of visuocortical investment to peripheral locations (Munoz and Everling, 2004). There was also a main effect of Time, F(4,148)=34.76, p=3.9E–26, with desynchronization increasing over time, suggesting that some decrease in peripheral investment may be important for both tasks, at least given the current stimulus paradigm. Both of these effects are consistent with previous findings using a similar task in healthy individuals (Clementz et al., 2010).
3.4. Inter-Trial Phase Coherence
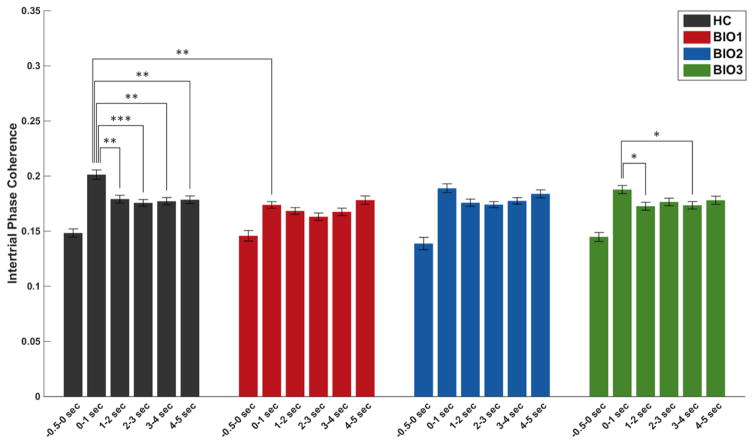
ITC showed a main effect of Time, F(5,148)=72.31, p=7.3E-62, with all time bins increased over baseline (Figure 4), and a Task × Time interaction, F(5,148)=2.35, p=.04, with greater ITC for anti- than pro-saccades at stimulus onset but the reverse effect later during the Preparatory Period. This is consistent with Clementz et al. (2010) and is expected, as the greater complexity of anti- versus pro-saccade tasks can yield greater initial involvement of dorsal visual stream circuitry (e.g., Dyckman et al., 2007; Ford et al., 2005), which is preferentially activated by peripheral stimuli (Ungerleider and Desimone, 1986). A Group × Time interaction, F(15,450)=1.8, p=.03, was driven by HC and Biotype-3 significantly decreasing ITC after stimulus onset, as well as Biotype-1 showing diminished ITC at stimulus onset. This neural synchronization decrease by HC and Biotype-3 may represent “gating” information flow from initially high down to levels more appropriate for successful performance (Steinmetz et al., 2000).
Figure 4.
Inter-Trial Phase Coherence. Inter-Trial Phase Coherence (ITC) averaged over the steady-state driving frequencies (12 and 15Hz). Bar plots represent ITC over visual cortex. For all groups, baseline ITC is significantly lower than all later time bins, though this is not indicated in the figure. Error bars indicate standard error of the mean. A mixed effects 3-way MANOVA (Group × Saccade Type × Time Bin) was calculated, with post hoc Tukey honestly significant difference tests on the estimated marginal means. *p<.05, **p<.01, ***p<.005
4. Discussion
Intrinsic neural activity levels differ between psychosis Biotypes, with activity being diminished in Biotype-1 and accentuated in Biotype-2. Despite this, examination of stimulus-specific oscillatory activity revealed no differences between groups. While all groups similarly modulated stimulus-specific neural power as a function of increasing cognitive demands, only HC and Biotype-1 modulated intrinsic activity. This pattern of results provides key implications for the understanding of cognitive control in psychosis and for the relationship between neurobiology and behavioral outcomes.
Individuals with psychotic disorders are often reported as showing deviant levels of non-specific neural activity (see Winterer and Weinberger, 2004), correspondingly deviant sensory cortical responses (Clementz et al., 2008; Spencer et al., 2004; Wang et al., 2010), and low signal-to-noise (Ethridge et al., 2011; Winterer et al., 2000). The current study found diametrically opposed abnormalities in intrinsic neural activity levels between psychosis subgroups, with Biotype-1 showing hypoactivity, Biotype-2 showing hyperactivity, and Biotype-3 the closest to HC, consistent with expectations based on Clementz et al. (2016). It may be that both Biotype-1 and Biotype-2 have “attractor state” (one of multiple, more-or-less stable firing patterns to which a given neural network can be attracted by internal or external cues) problems, although for different reasons (Rolls et al., 2008). Neuronal hyperexcitation, as in Biotype-2, would decrease the stability of cortical networks selected to support current behavioral requirements. Competing cortical networks could periodically attain higher signal strength, leading to behavioral indications of “distraction.” Neuronal hypoexcitation, as in Biotype-1, could result in difficulty initiating switches between cortical network states, which could also lead to many of the cognitive symptoms found in psychosis, including reduced behavioral flexibility (Ethridge et al., 2014; Rolls et al., 2008). The hypoexcitation of Biotype-1 and hyperexcitation of Biotype-2 may be consistent with models of psychosis that invoke NMDA-receptor dysfunction on inhibitory interneurons (Javitt, 2007; Rujescu et al., 2006) or models of psychosis with the NMDA-receptor dysfunction on pyramidal neurons themselves (Li et al., 2015), but association with these potential mechanistic underpinnings remains to be established.
The current findings support a proposal by Clementz et al. (2016) that exploration into potassium and/or calcium channel alterations and channel-based therapies which may correct neuronal hypo- or hyperexcitability (Yanagi et al., 2014) might be fruitful for both subgroups. As either abnormally high or low NMDA-related current flow could decrease cortical network stability (Rolls et al., 2008), it is noteworthy that HC and Biotype-3 showed greater and more dynamic changes in neural synchrony than Biotype-1 or Biotype-2, suggesting an inability in the latter groups to properly modulate synchronous activity. It is unclear why ITC deviations in Biotypes-1 and -2 were found only after trial onset in the current study and only before trial onset in Hudgens-Haney et al. (2017). One possibility may be differential processing of the central versus peripheral steady-state stimuli (Stephen et al., 2002), which could also provide insight into why Biotype-2 exhibited better antisaccade performance in the current study than in Hudgens-Haney et al. (2017). Further study will be needed on the heterogeneity among psychosis subgroups of central versus peripheral visual processing pathways and susceptibility to distraction under varying stimulus and task conditions.
Findings from studies on nonhuman primates (Munoz and Everling, 2004) and HC (Clementz et al., 2010) indicate that successful antisaccade performance requires sustained anticipatory modulation of neural activity in specific regions of saccade motor circuitry, presumably via top-down control by prefrontal cortex (Johnston and Everling, 2006). Together with findings from Hudgens-Haney et al. (2017), the current results provide direct evidence in the same sample of HC for the complementary processes of increasing visuocortical investment to central stimuli and decreasing investment to peripheral stimuli prior to anti- relative to pro-saccades. Interestingly, both of these preparatory neural modulation processes were found to be preserved in psychosis Biotypes, despite diminished behavioral performance. This finding indicates that preparatory modulation alone is not sufficient for successful antisaccade performance. While all groups similarly modulated stimulus-specific neural power as a function of increasing cognitive demands, only HC and Biotype-1 modulated intrinsic activity. That Biotype-2 and Biotype-3, both with slightly impaired behavioral performance, failed to modulate intrinsic activity levels as a function of cognitive demands suggests differences in the cognitive strategies employed by each group. For Biotype-1, however, increases in task-specific neural activity appear to require large increases in non-specific activity. This may indicate an attempt to compensate for overall hypoactivity and/or a propensity to become overwhelmed as cognitive demands increase.
Distinct subgroups of psychosis cases show diametrically opposed deviations in nonspecific neural oscillatory activity. This pattern of differences between groups in absolute activity levels is also reflected in evoked potentials at stimulus onset (see Figure 2A). It is unlikely that these differences are the result of medication effects, as Biotypes were similarly medicated (see Table 1). While the results of the current study advance the notion that differences in intrinsic neural activity levels play a key role in cognitive performance in psychosis, they also indicate that intrinsic activity levels cannot fully explain the differences between psychosis subgroups or vs. HC. For example, Biotype-3 did not deviate significantly from HC on any brain measure, despite slightly impaired behavioral performance. Finally, replication of all analyses subgrouping psychosis cases based on either DSM diagnosis or a continuum of psychotic symptomology revealed fewer and less consistent differences, including no intrinsic activity differences between any clinical subgroup and healthy individuals (see Supplemental Results). While not the principle interest of the current investigation, it is nonetheless important to highlight this finding. The reproducibility and longitudinal stability of the Biotype constructs developed in Clementz et al. (2016) are being addressed in independent samples in our ongoing work, but it is clear that there exist meaningful differences between distinct psychosis subgroups that are not adequately accounted for by clinical symptomatology. Above all, these findings indicate that grouping patients based on multidimensional classification using neurobiological data has demonstrable advantages for resolving heterogeneity and clarifying illness mechanisms relative to traditional psychiatric diagnoses and emphasize the need for studies of psychosis to consider the possibility of complex interactions between behavioral and brain measures.
Supplementary Material
Acknowledgments
Role of the Funding Source
Funding for this study was provided by National Institutes of Health Grants MH077862 and MH085485. The funding source played no role in data analysis or interpretation.
The authors would like to acknowledge the National Institutes of Health for providing funding for this project.
Footnotes
Contributors
BAC and JAS designed the study and wrote the protocol. SKK collected the data. LEE performed preliminary analyses. MEH performed all final analyses. MEH and BAC wrote the manuscript. All authors edited the manuscript and have seen and approved the final version.
Financial Disclosures
Author JAS has served as a consultant for Takeda, Inc. The remaining authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Carroll CA, Kieffaber PD, Vohs JL, O’Donnell BF, Shekhar A, Hetrick WP. Contributions of spectral frequency analyses to the study of P50 ERP amplitude and suppression in bipolar disorder with or without a history of psychosis. Bipolar Disord. 2008;10:776–787. doi: 10.1111/j.1399-5618.2008.00622.x. [DOI] [PubMed] [Google Scholar]
- Clementz BA, Blumenfeld L. Multichannel electroencephalographic assessment of auditory evoked response suppression in schizophrenia. Exp Brain Res. 2001;139:377–390. doi: 10.1007/s002210100744. [DOI] [PubMed] [Google Scholar]
- Clementz BA, Brahmbhatt SB, McDowell JE, Brown R, Sweeney JA. When does the brain inform the eyes whether and where to move? An EEG study in humans. Cereb Cortex. 2007;17:2634–43. doi: 10.1093/cercor/bhl171. [DOI] [PubMed] [Google Scholar]
- Clementz BA, Gao Y, McDowell JE, Moratti S, Keedy SK, Sweeney JA. Top-down control of visual sensory processing during an ocular motor response inhibition task. Psychophysiology. 2010;47:1011–8. doi: 10.1111/j.1469-8986.2010.01026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clementz BA, Sponheim SR, Iacono WG, Beiser M. Resting EEG in first-episode schizophrenia patients, bipolar psychosis patients, and their first-degree relatives. Psychophysiology. 1994;31:486–94. doi: 10.1111/j.1469-8986.1994.tb01052.x. [DOI] [PubMed] [Google Scholar]
- Clementz BA, Sweeney JA, Hamm JP, Ivleva EI, Ethridge LE, Pearlson GD, Keshavan MS, Tamminga CA. Identification of distinct psychosis biotypes using brain-based biomarkers. Am J Psychiatry. 2016;173:373–384. doi: 10.1176/appi.ajp.2015.14091200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clementz BA, Wang J, Keil A. Normal electrocortical facilitation but abnormal target identification during visual sustained attention in schizophrenia. J Neurosci. 2008;28:13411. doi: 10.1523/JNEUROSCI.4095-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Russo F, Taddei F, Apnile T, Spinelli D. Neural correlates of fast stimulus discrimination and response selection in top-level fencers. Neurosci Lett. 2006;408:113–8. doi: 10.1016/j.neulet.2006.08.085. [DOI] [PubMed] [Google Scholar]
- Dien J. Applying Principal Components Analysis to Event-Related Potentials: A Tutorial. Dev Neuropsychol. 2012;37:497–517. doi: 10.1080/87565641.2012.697503. [DOI] [PubMed] [Google Scholar]
- Dyckman KA, Camchong J, Clementz BA, McDowell JE. An effect of context on saccade-related behavior and brain activity. Neuroimage. 2007;36:774–784. doi: 10.1016/j.neuroimage.2007.03.023. [DOI] [PubMed] [Google Scholar]
- Ethridge LE, Hamm JP, Pearlson GD, Tamminga CA, Sweeney JA, Keshavan MS, Clementz BA. Event-Related Potential and Time-Frequency Endophenotypes for Schizophrenia and Psychotic Bipolar Disorder. Biol Psychiatry. 2015;77:127–136. doi: 10.1016/j.biopsych.2014.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ethridge LE, Moratti S, Gao Y, Keil A, Clementz Ba. Sustained versus transient brain responses in schizophrenia: the role of intrinsic neural activity. Schizophr Res. 2011;133:106–11. doi: 10.1016/j.schres.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ethridge LE, Soilleux M, Nakonezny PA, Reilly JL, Kristian Hill S, Keefe RSE, Gershon ES, Pearlson GD, Tamminga CA, Keshavan MS, Sweeney JA. Behavioral response inhibition in psychotic disorders: Diagnostic specificity, familiality and relation to generalized cognitive deficit. Schizophr Res. 2014;159:491–498. doi: 10.1016/j.schres.2014.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV Axis I Disorders. American Psychiatric Publishing; Arlington, VA, VA: 1997. [Google Scholar]
- Ford KA, Goltz HC, Brown MRG, Everling S, Kristen A. Neural Processes Associated With Antisaccade Task Performance Investigated With Event-Related fMRI. J Neurophysiol. 2005;94:429–440. doi: 10.1152/jn.00471.2004. [DOI] [PubMed] [Google Scholar]
- Hallett PE. Primary and secondary saccades to goals defined by instructions. Vision Res. 1978;18:1279–1296. doi: 10.1016/0042-6989(78)90218-3. [DOI] [PubMed] [Google Scholar]
- Hamm JP, Ethridge LE, Boutros NN, Keshavan MS, Sweeney JA, Pearlson GD, Tamminga CA, Clementz BA. Diagnostic specificity and familiality of early versus late evoked potentials to auditory paired stimuli across the schizophrenia-bipolar psychosis spectrum. Psychophysiology. 2014;51:348–357. doi: 10.1111/psyp.12185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudgens-Haney ME, Ethridge LE, Knight JB, McDowell JE, Keedy SK, Pearlson GD, Tamminga CA, Keshavan MS, Sweeney JA, Clementz BA. Intrinsic neural activity differences among psychotic illnesses. Psychophysiology. 2017 doi: 10.1111/psyp.12875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC. Glutamate and Schizophrenia: Phencyclidine, N-Methyl-d-Aspartate Receptors, and Dopamine–Glutamate Interactions. Int Rev Neurobiol. 2007;78:69–108. doi: 10.1016/S0074-7742(06)78003-5. [DOI] [PubMed] [Google Scholar]
- Johnston K, Everling S. Neural Activity in Monkey Prefrontal Cortex Is Modulated by Task Context and Behavioral Instruction during Delayed-match-to-sample and Conditional Prosaccade—Antisaccade Tasks. J Cogn Neurosci. 2006;18:749–765. doi: 10.1162/jocn.2006.18.5.749. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Clementz Ba, Pearlson GD, Sweeney Ja, Tamminga Ca. Reimagining psychoses: An agnostic approach to diagnosis. Schizophr Res. 2013;146:10–6. doi: 10.1016/j.schres.2013.02.022. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Morris DW, Sweeney JA, Pearlson GD, Thaker G, Seidman LJ, Eack SM, Tamminga CA. A dimensional approach to the psychosis spectrum between bipolar disorder and schizophrenia: The Schizo-Bipolar Scale. Schizophr Res. 2011;133:250–254. doi: 10.1016/j.schres.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan GP, Vohs JL, Hetrick WP, Carroll CA, Shekhar A, Bockbrader MA, O’Donnell BF. Steady state visual evoked potential abnormalities in schizophrenia. Clin Neurophysiol. 2005;116:614–24. doi: 10.1016/j.clinph.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Li W, Ghose S, Gleason K, Begovic A, Perez J, Bartko J, Russo S, Wagner AD, Selemon L, Tamminga CA. Synaptic proteins in the hippocampus indicative of increased neuronal activity in CA3 in schizophrenia. Am J Psychiatry. 2015;172:373–382. doi: 10.1176/appi.ajp.2014.14010123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauchly JW. Significance Test for Sphericity of a Normal n-Variate Distribution. Ann Math Stat. 1940;11:204–209. [Google Scholar]
- McDowell JE, Dyckman KA, Austin BP, Clementz BA. Neurophysiology and neuroanatomy of reflexive and volitional saccades: Evidence from studies of humans. Brain Cogn. 2008;68:255–270. doi: 10.1016/j.bandc.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell JE, Kissler JM, Berg P, Dyckman KA, Gao Y, Rockstroh B, Clementz BA. Electroencephalography/magnetoencephalography study of cortical activities preceding prosaccades and antisaccades. Neuroreport. 2005;16:663–668. doi: 10.1097/00001756-200505120-00002. [DOI] [PubMed] [Google Scholar]
- McDowell JE, Myles-Worsley M, Coon H, Byerley W, Clementz BA. Measuring liability for schizophrenia using optimized antisaccade stimulus parameters. Psychophysiology. 1999;36:138–141. doi: 10.1017/S0048577299980836. [DOI] [PubMed] [Google Scholar]
- Moratti S, Clementz BA, Gao Y, Ortiz T, Keil A. Neural mechanisms of evoked oscillations: stability and interaction with transient events. Hum Brain Mapp. 2007;28:1318–33. doi: 10.1002/hbm.20342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz DP, Everling S. Look away: the anti-saccade task and the voluntary control of eye movement. Nat Rev Neurosci. 2004;5:218–28. doi: 10.1038/nrn1345. [DOI] [PubMed] [Google Scholar]
- Pastor MA, Artieda J, Arbizu J, Valencia M, Masdeu JC. Human Cerebral Activation during Steady-State Visual-Evoked Responses. J Neurosci. 2003;23:11621–11627. doi: 10.1523/JNEUROSCI.23-37-11621.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly JL, Frankovich K, Hill S, Gershon ES, Keefe RSE, Keshavan MS, Pearlson GD, Tamminga CA, Sweeney JA. Elevated antisaccade error rate as an intermediate phenotype for psychosis across diagnostic categories. Schizophr Bull. 2014;40:1011–21. doi: 10.1093/schbul/sbt132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET, Loh M, Deco G, Winterer G. Computational models of schizophrenia and dopamine modulation in the prefrontal cortex. Nat Rev Neurosci. 2008;9:696–709. doi: 10.1038/nrn2462. [DOI] [PubMed] [Google Scholar]
- Rujescu D, Bender A, Keck M, Hartmann AM, Ohl F, Raeder H, Giegling I, Genius J, McCarley RW, Möller HJ, Grunze H. A Pharmacological Model for Psychosis Based on N-methyl-D-aspartate Receptor Hypofunction: Molecular, Cellular, Functional and Behavioral Abnormalities. Biol Psychiatry. 2006;59:721–729. doi: 10.1016/j.biopsych.2005.08.029. [DOI] [PubMed] [Google Scholar]
- Spencer KM, Nestor PG, Perlmutter R, Niznikiewicz Ma, Klump MC, Frumin M, Shenton ME, McCarley RW. Neural synchrony indexes disordered perception and cognition in schizophrenia. Proc Natl Acad Sci U S A. 2004;101:17288–93. doi: 10.1073/pnas.0406074101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz PN, Roy a, Fitzgerald PJ, Hsiao SS, Johnson KO, Niebur E. Attention modulates synchronized neuronal firing in primate somatosensory cortex. Nature. 2000;404:187–190. doi: 10.1038/35004588. [DOI] [PubMed] [Google Scholar]
- Stephen JM, Aine CJ, Christner RF, Ranken D, Huang M, Best E. Central versus peripheral visual field stimulation results in timing differences in dorsal stream sources as measured with MEG. Vision Res. 2002;42:3059–3074. doi: 10.1016/S0042-6989(02)00415-7. [DOI] [PubMed] [Google Scholar]
- Sweeney JA, Mintun MA, Kwee S, Wiseman MB, Brown DL, Rosenberg DR, Carl JR. Positron emission tomography study of voluntary saccadic eye movements and spatial working memory. J Neurophysiol. 1996;75:454–468. doi: 10.1152/jn.1996.75.1.454. [DOI] [PubMed] [Google Scholar]
- Tamminga CA, Ivleva EI, Keshavan MS, Pearlson GD, Clementz BA, Witte B, Morris DW, Bishop JR, Thaker GK, Sweeney JA. Clinical phenotypes of psychosis in the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) Am J Psychiatry. 2013;170:1263–74. doi: 10.1176/appi.ajp.2013.12101339. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Desimone R. Projections to the superior temporal sulcus from the central and peripheral field representations of V1 and V2. J Comp Neurol. 1986;248:147–163. doi: 10.1002/cne.902480202. [DOI] [PubMed] [Google Scholar]
- Wang J, Brown R, Dobkins KR, McDowell JE, Clementz BA. Diminished parietal cortex activity associated with poor motion direction discrimination performance in schizophrenia. Cereb Cortex. 2010;20:1749–1755. doi: 10.1093/cercor/bhp243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Clementz BA, Keil A. The neural correlates of feature-based selective attention when viewing spatially and temporally overlapping images. Neuropsychologia. 2007;45:1393–1399. doi: 10.1016/j.neuropsychologia.2006.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterer G, Coppola R, Goldberg TE, Egan MF, Jones DW, Sanchez CE, Weinberger DR. Prefrontal Broadband Noise, Working Memory, and Genetic Risk for Schizophrenia. Am J Psychiatry. 2004;161:490–500. doi: 10.1176/appi.ajp.161.3.490. [DOI] [PubMed] [Google Scholar]
- Winterer G, Musso F, Beckmann C, Mattay V, Egan MF, Jones DW, Callicott JH, Coppola R, Weinberger DR. Instability of prefrontal signal processing in schizophrenia. Am J Psychiatry. 2006;163:1960–1968. doi: 10.1176/appi.ajp.163.11.1960. [DOI] [PubMed] [Google Scholar]
- Winterer G, Weinberger DR. Genes, dopamine and cortical signal-to-noise ratio in schizophrenia. Trends Neurosci. 2004;27:683–690. doi: 10.1016/j.tins.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Winterer G, Ziller M, Dorn H, Frick K, Mulert C, Wuebben Y, Herrmann W, Coppola R. Schizophrenia: reduced signal-to-noise ratio and impaired phase-locking during information processing. Clin Neurophysiol. 2000;111:837–849. doi: 10.1016/S1388-2457(99)00322-3. [DOI] [PubMed] [Google Scholar]
- Yanagi M, Joho RH, Southcott SA, Shukla AA, Ghose S, Tamminga CA. Kv3.1-containing K(+) channels are reduced in untreated schizophrenia and normalized with antipsychotic drugs. Mol Psychiatry. 2014;19:573–9. doi: 10.1038/mp.2013.49. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.