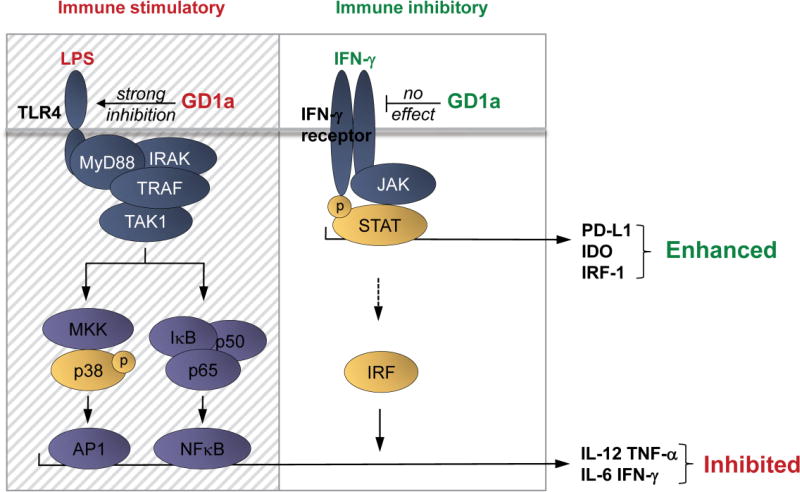
Figure 5. Complex interaction of TLR-ligation, ganglioside enrichment, and IFN-γ on dendritic cell signaling.
LPS/TLR-4 mediated activation of p38 drives the secretion of pro-inflammatory factors such as IL-12, TNF-α, IL-6 but also IFN-γ, through active AP1. This also involves the translocation of NFκB transcription factors into the nucleus promoting cytokine production. IFN-γ, by inducing JAK/STAT mediated signal transduction triggers the expression of IRFs, which boost cytokine production in combination with AP1 and NFκB transcription factors. IRF-1 itself is not capable of inducing cytokine production as we have also shown here. IFN-γ as well triggers transcription of IDO1 and PD-L1, inducing an immunosuppressive DC phenotype. In a ganglioside-rich environment, GD1a accumulation in DCs specifically inhibits TLR-4 signaling, strongly reducing p38-mediated AP1 activity and NFκB activity, leading to down modulation of pro-inflammatory cytokine secretion. Of note, IFN-γ signaling is not affected by GD1a enrichment, leaving JAK/STAT-mediated PD-L1 and IDO1 induction by IFN-γ intact. GD1a enriched environments therefore strongly favor immune suppressive properties of DCs in the presence of IFN-γ, which predominantly triggers the expression of enzymatically active IDO1 and PD-L1. In the figure, the hatched area indicates selective inhibitory effects of GD1a on TLR-4 signaling; the clear area depicts the unaffected IFN-γ signaling. TRAF:TNF receptor associated factor, TAK1: Transforming growth factor beta-activated kinase 1, AP1: Activator protein 1, NFκB: Nuclear factor kappa-light-chain-enhancer of activated B cells, IRF: Interferon regulatory factors.