Abstract
Alcohol intake has been associated with lung function levels inconsistently in cross-sectional studies. The goal of our study was to determine whether longitudinally-assessed light-to-moderate alcohol intake is associated with levels and decline of lung function. We examined data from 1,333 adult participants in the population-based Tucson Epidemiological Study of Airway Obstructive Disease. Alcohol intake was assessed at four surveys between 1972 and 1992. Subjects who completed at least two surveys were classified into longitudinal drinking categories (“never”, “inconsistent”, or “persistent drinker”). Spirometric lung function was measured in up to 11 surveys between 1972 and 1992. Random coefficient models were used to test for differences in lung function by drinking categories. After adjustment for sex, age, height, education, BMI categories, smoking status, and pack-years, as compared to never drinkers, persistent drinkers had higher FVC (coefficient: 157 ml, p<0.001), but lower FEV1/FVC ratio (−2.3%, p<0.001). Differences were due to a slower decline of FVC among persistent than never drinkers (p=0.003) and these trends were present independent of smoking status. Inconsistent drinking showed similar, but weaker associations. After adjustment for potential confounders, light-to-moderate alcohol consumption was associated with a significantly decreased rate of FVC decline over adult life.
Keywords: alcohol, lung function, restriction, obstruction
INTRODUCTION
Several studies have found that moderate alcohol intake is associated with increased lung function measures – particularly forced vital capacity (FVC) and forced expiratory volume in one second (FEV1) – in the general population, but these findings have not always been consistent even after taking into account possible confounders like concomitant cigarette smoking (Cohen et al., 1980; Frantz, Wollmer, Dencker, Engstrom, & Nihlen, 2014; Garshick, Segal, Worobec, Salekin, & Miller, 1989; Lange et al., 1988; Root, Houser, Anderson, & Dawson, 2014; Schunemann et al., 2002; Sisson et al., 2005; Siu, Udaltsova, Iribarren, & Klatsky, 2010; Sparrow, Rosner, Cohen, & Weiss, 1983; Tabak, Smit, Heederik, Ocke, & Kromhout, 2001; Tabak, Smit, Rasanen, et al., 2001; Twisk, Staal, Brinkman, Kemper, & van Mechelen, 1998; Zureik, Liard, Kauffmann, Henry, & Neukirch, 1996). A possible explanation for these discrepancies is that the relation of alcohol intake to lung function is largely dependent on both the quantity and duration of alcohol consumption. Indeed, studies have described a U-shaped (Tabak, Smit, Rasanen, et al., 2001) or J-shaped (Siu et al., 2010) relationship in which the association between alcohol and lung function was protective for light to moderate consumers but harmful for heavy consumers.
Longitudinal evaluation is critical in determining both the protective and deleterious impact of environmental and behavioral factors on lung function. Similarly, assessing the duration of drinking requires the availability of longitudinal data gathered frequently over a significant period of time to minimize the risk of recall bias. To date only a few prospective studies (Lange et al., 1988; Root et al., 2014; Sparrow et al., 1983; Twisk et al., 1998; Zureik et al., 1996) have examined the effects of alcohol consumption on longitudinal measures of lung function, but no previous study has investigated the effects of longitudinal patterns of alcohol consumption (i.e., persistent vs. inconsistent vs. never drinking) on levels and trajectories of lung function. In this study, we sought to examine the relation of longitudinal patterns of alcohol intake on lung function in a prospective design using the long-term population-based cohort of the Tucson Epidemiological Study of Airway Obstructive Disease (TESAOD).
MATERIALS AND METHODS
Study population
The TESAOD cohort is a population-based prospective cohort study of non-Hispanic white households that was initiated in Tucson, AZ in 1972. Details of the enrollment process have been previously reported (Lebowitz, Knudson, & Burrows, 1975). At enrollment, TESAOD participants completed a standardized respiratory questionnaire and performed spirometric lung function tests with a pneumotachygraph according to methods previously described (Knudson, Slatin, Lebowitz, & Burrows, 1976). Spirometry was used to measure FVC, the volume of air that an individual can forcefully exhale after a full inhalation, and FEV1, the volume of air that an individual can forcefully exhale in the first second of the maneuver (Pellegrino et al., 2005). Reduction in the FEV1/FVC ratio demonstrates an obstructive impairment of lung function (Pellegrino et al., 2005). Twelve additional follow-up surveys were completed approximately every two years up to 1996. Lung function tests were completed in all surveys - with the only exception of survey 4 - using the American Thoracic Society criteria.
Longitudinal Drinking Categories
Questions regarding alcohol intake were asked in four surveys (1, 7, 11, and 12). The 1st survey was administered in 1972–73, the 7th survey in 1981–83, the 11th survey in 1988–89, and the 12th survey in 1990–92. At each of these four surveys, subjects were asked to report YES or NO to the following question: “Do you drink any alcohol?” Subjects reporting drinking alcohol were then asked what amount of wine, beer, and hard liquor they drank on average per week. Drinks were defined as one glass of wine, one glass of beer, or one shot of hard liquor. We defined a longitudinal categorical drinking variable based on the responses provided during the four surveys. Only subjects who completed at least two of the four surveys when they were ≥ 21 years old were included in the analysis. Due to the potentially damaging effects of heavy alcohol consumption on the lung, subjects who at any survey reported heavy alcohol intake, defined as having more than 140 drinks per month were excluded from primary analyses. Sensitivity analyses were also completed and main results confirmed 1) after considering an alternate heavy drinking threshold that excluded subjects reporting more than 90 drinks per month (Tables E1–E3), and 2) after completing analyses on all participants, including heavy drinkers (Tables E4–E6). Subjects were classified as a “never drinker” if they reported “No” to the alcohol question in all completed surveys. Subjects were classified as an “inconsistent drinker” if they reported both YES and NO in different surveys. Subjects were classified as a “persistent drinker” if they always reported YES. The “never drinkers” were considered the reference category.
Longitudinal Drinking and Smoking Categories
Similarly to what was done for alcohol intake, we also categorized smoking status longitudinally and classified subjects into combined longitudinal drinking and smoking categories. Although smoking was assessed in all surveys, for consistency we only considered smoking status from the four surveys in which alcohol intake was also assessed. Based on data from these four surveys, we classified subjects into five categories as: 1) “never drinker and never smoker”; 2) “persistent drinker and never smoker”; 3) “never drinker and persistent smoker”; 4) “persistent drinker and persistent smoker”; and 5) “inconsistent drinker or smoker”. Subjects who reported inconsistent drinking regardless of their smoking status, inconsistent smoking regardless of their alcohol status, or subjects who reported former smoking at each of the four surveys were classified in the “inconsistent drinker or smoker” group. The “never drinkers and never smokers” were considered the reference category.
Quantitative Drinking Exposure
Similar to previous work (Sisson et al., 2005), we also assessed quantitative alcohol intake and generated categories by the average number of drinks per month. In each of the four surveys, subjects were classified by their average number of drinks per month in six categories: 1) “none”; 2) “<5”; 3) “5 to <15”; 4) “15 to <30”; 5) “30 to <90”; and 6) “90 to <140” drinks per month.
Covariates
At the time of the spirometric test, study nurses measured subjects’ height and weight. Body mass index (BMI) was computed and BMI categories were defined as underweight (<18.5 Kg/m2), normal weight (≥18.5 and <25), overweight (≥25 and <30), and obese (≥30). Information on usual number of cigarettes smoked per day, age when the subject began to smoke, and age when the subject quit smoking was collected at each of the four surveys and used to compute pack-years for smokers. Education was assessed at surveys 1, 9, and 12. Maximum education attained from these surveys was defined as less than or equal to a high school education (≤12 years) and greater than a high school education (>12 years).
Longitudinal Statistical Analysis
The primary goal of statistical analyses was to test for differences in lung function levels and/or decline associated with persistent drinking, both adjusted for and in combination with concurrent smoking. Since multiple lung function observations were available for each subject, we used Random Coefficient Models (RCMs) to model the data. RCMs adjust for the serial correlation between repeated observations within subjects (Brown & Prescott, 2001). The variance matrix of these random coefficients was modeled assuming an “unstructured pattern”. Only lung function tests completed at ≥ 21 years and up to survey 12 were included in these models. Separate models were run (1) with the longitudinal drinking categories and (2) with the longitudinal drinking and smoking categories as the main independent variables of interest. These variables were both tested as main effects to evaluate their association with levels of lung function and as interaction terms with age to evaluate their effects on lung function decline. In addition, sex, age, height, pack-years, education, and BMI categories were included as covariates in all models. Separate RCMs were used to predict FVC, FEV1, and their ratio FEV1/FVC. A final analysis was also completed in which multinomial logistic regression models – which included the variance estimate component option with cluster to adjust for serial correlation – were used to model the effect of the longitudinal drinking categories on spirometric restriction and obstruction. For this analysis, at each survey participants were categorized into one of three mutually exclusive lung function groups:
“Spirometric Restriction” defined as FEV1/FVC ≥ 70% plus FVC % predicted < 80%
“Spirometric Obstruction” defined as FEV1/FVC < 70% regardless of FVC levels
“Normal” defined as FEV1/FVC ≥ 70% and FVC % predicted ≥ 80%
Statistical analyses were performed with Stata version 14.0 (StataCorp LP, College Station, TX, USA).
RESULTS
Characteristics of participants
Table 1 shows the characteristics of the TESAOD participants who – in at least two of the four surveys in which alcohol questions were asked – were at least 21 years of age, had available information on alcohol intake, were not heavy drinkers, and completed lung function tests. Overall, analyses included 1,333 individual participants and a total of 10,264 lung function observations over a total of 11 surveys. Among the 1,333 participants, 360 (27.0%) reported never drinking, 352 (26.4%) reported inconsistent drinking, and 621 (46.6%) reported persistent drinking. Percentage of participants who reported alcohol intake slightly decreased over surveys 1, 7, 11, and 12 (64.2%, 59.4%, 59.5%, 58.2% respectively) with a greater decrease in report of current smoking (31.7%, 23.5%, 19.1%, 17.6% respectively). Also, the prevalence of obesity increased over time (6.6%, 12.2%, 13.5%, 14.3% respectively). The mean age at each survey was 49, 55, 55, and 56 years with 60%–62% female participation.
Table 1.
Characteristics of subjects (overall N=1,333) who provided information on alcohol intake in at least 2 surveys from Survey 1, 7, 11, 12.
| Survey 1 (N=952) | Survey 7 (N=1061) | Survey 11 (N=939) | Survey 12 (N=865) | |||||
|---|---|---|---|---|---|---|---|---|
| Continuous Variables | Mean | SD | Mean | SD | Mean | SD | Mean | SD |
| Age (yrs) | 48.83 | 16.04 | 55.51 | 18.00 | 55.00 | 18.14 | 56.24 | 17.68 |
| Height (in) | 65.62 | 3.64 | 65.52 | 3.72 | 65.85 | 3.77 | 65.90 | 3.81 |
| FEV1 (L) | 2.91 | 0.92 | 2.89 | 1.04 | 2.92 | 1.00 | 2.83 | 0.99 |
| FVC (L) | 3.62 | 1.08 | 3.78 | 1.22 | 3.79 | 1.18 | 3.70 | 1.17 |
| FEV1/FVC (%) | 80.30 | 9.00 | 76.19 | 9.51 | 76.64 | 9.09 | 76.07 | 8.93 |
| Pack-Years1 | ||||||||
| All | 12.71 | 18.94 | 16.06 | 22.80 | 14.88 | 21.27 | 14.93 | 21.35 |
| Among ever smokers | 22.59 | 20.36 | 28.80 | 23.92 | 30.22 | 23.07 | 33.20 | 22.58 |
| Categorical Variables | Frequency | Percent | Frequency | Percent | Frequency | Percent | Frequency | Percent |
| Lung function groups | ||||||||
| Normal | 749 | 78.68 | 804 | 75.78 | 727 | 77.42 | 648 | 74.91 |
| Restriction | 99 | 10.40 | 43 | 4.05 | 38 | 4.05 | 44 | 5.09 |
| Obstruction | 104 | 10.92 | 214 | 20.17 | 174 | 18.53 | 173 | 20.00 |
| BMI2 | ||||||||
| Normal | 531 | 57.59 | 518 | 48.82 | 470 | 50.16 | 398 | 46.01 |
| Underweight | 17 | 1.84 | 24 | 2.26 | 20 | 2.13 | 26 | 3.01 |
| Overweight | 313 | 33.95 | 390 | 36.76 | 321 | 34.26 | 317 | 36.65 |
| Obese | 61 | 6.62 | 129 | 12.16 | 126 | 13.45 | 124 | 14.34 |
| Sex (F) | 585 | 61.45 | 639 | 60.23 | 577 | 61.45 | 535 | 61.85 |
| Drink (any) | 611 | 64.18 | 630 | 59.38 | 559 | 59.53 | 503 | 58.15 |
| Drinking exposure categories3 | ||||||||
| None | 341 | 35.89 | 431 | 42.13 | 380 | 40.82 | 362 | 41.95 |
| <5 | 137 | 14.42 | 103 | 10.07 | 172 | 18.47 | 218 | 25.26 |
| 5–15 | 154 | 16.21 | 152 | 14.86 | 117 | 12.57 | 68 | 7.88 |
| 15–30 | 168 | 17.68 | 152 | 14.86 | 144 | 15.47 | 108 | 12.51 |
| 30–90 | 137 | 14.42 | 171 | 16.72 | 110 | 11.82 | 96 | 11.12 |
| 90–140 | 13 | 1.37 | 14 | 1.37 | 8 | 0.86 | 11 | 1.27 |
| Smoke Status4 | ||||||||
| Current | 291 | 31.68 | 249 | 23.49 | 179 | 19.08 | 152 | 17.63 |
| Ex | 245 | 25.69 | 359 | 33.87 | 357 | 38.06 | 302 | 35.03 |
| Never | 415 | 42.64 | 452 | 42.64 | 402 | 42.86 | 408 | 47.33 |
Pack-years missing (N=3: Survey 1, N=7: Survey 7, N=16: Survey 11, N=8: Survey 12)
BMI missing (N=30: Survey 1, N=2: Survey 11)
Drinking exposure missing (N=2: Survey 1, N=38: Survey 7, N=8: Survey 11, N=2: Survey 12)
Smoke status missing (N=1: Survey 1, N=1: Survey 7, N=1: Survey 11, N=3: Survey 12)
There were significant differences in the characteristics of participants across the drinking categories, with persistent drinkers being younger and more likely to be males and smokers than never drinkers (Table E7). In addition, drinking patterns were associated with education, with 43% of never drinkers, 52% of intermittent drinkers, and 67% of persistent drinkers reporting greater than 12 years of education (p<0.001).
The relation of persistent alcohol intake to lung function
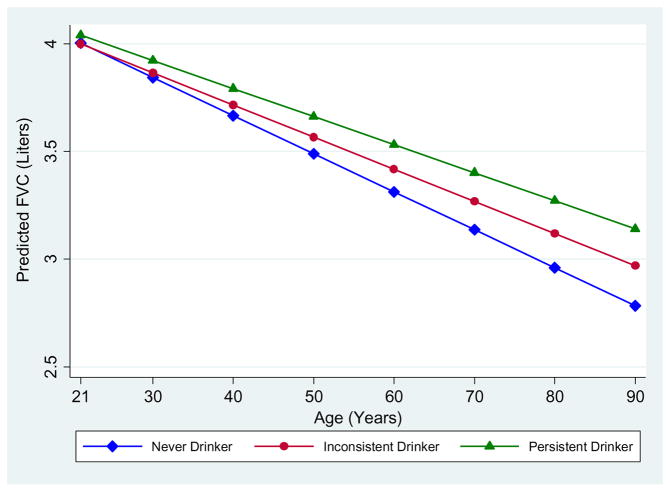
In random coefficients models, persistent drinking was associated with increased FVC levels, after adjusting for sex, age, height, smoking, pack-years, education, and BMI categories (upper part of Table 2). As compared to never drinkers, persistent drinkers had FVC levels across adult life that were on average 157 ml higher (p<0.001) and inconsistent drinkers had a non-significant FVC increase of 74 ml (0.126). Neither persistent nor inconsistent drinkers had FEV1 levels that differed from those of never drinkers. In contrast, as compared to never drinkers, the FEV1/FVC ratio was on average 2.3% lower (p<0.001) for persistent drinkers and 1.4% lower (p=0.020) for inconsistent drinkers. Results from models including an interaction term with age (lower part of Table 2) demonstrated that the FVC differences were due to a significantly slower decline of FVC among persistent drinkers as compared with never drinkers (p=0.003). As shown in Figure 1, FVC levels at age 21 years were comparable between never and persistent drinkers but their decline during adult life was 5 ml/yr slower for the latter group. Similar, albeit non-significant (p=0.109) trends of slower FVC decline were found for the group of inconsistent drinkers.
Table 2.
Random Coefficient Model (RCM) results with persistent drinking categories (N=1,333 subjects with 10,264 total observations)
| FVC in ml | FEV1 in ml | FEV1/FVC in % | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Coefficient | SE | P value | Coefficient | SE | P value | Coefficient | SE | P value | |
| MAIN EFFECTS | |||||||||
| Persistent Drink categories* | |||||||||
| Never (ref) | |||||||||
| Inconsistent | 73.96 | 48.35 | 0.126 | 8.84 | 42.22 | 0.834 | −1.37 | 0.59 | 0.020 |
| Persistent | 156.59 | 43.99 | <0.001 | 42.07 | 38.46 | 0.274 | −2.30 | 0.54 | <0.001 |
| MAIN EFFECTS + INTERACTIONS ** | |||||||||
| Persistent Drink categories* | |||||||||
| Never (ref) | |||||||||
| Inconsistent | −5.21 | 67.39 | 0.938 | −41.21 | 58.48 | 0.481 | −1.12 | 0.99 | 0.260 |
| Persistent | 36.28 | 60.11 | 0.546 | 21.58 | 52.28 | 0.680 | −1.32 | 0.88 | 0.133 |
| Inconsistent X Age | 2.80 | 1.75 | 0.109 | 1.85 | 1.49 | 0.214 | −0.01 | 0.03 | 0.777 |
| Persistent X Age | 4.66 | 1.58 | 0.003 | 0.72 | 1.34 | 0.593 | −0.03 | 0.02 | 0.147 |
Adjusted for sex, age, height, smoking category, pack-years, BMI categories, education category
Age was centered at 21 years, so in these models main effects refer to associations of drinking categories with lung function at age 21 years
Figure 1.
Predicted levels and decline of FVC from age 21 years onward across persistent drinking categories in TESAOD
Lines represent predicted values across the drinking categories for a non smoking female, with a height of 168 cm, normal body mass index, and more than a high school education.
The relation of persistent alcohol intake and smoking to lung function
When considering the combination of alcohol intake and smoking in fully adjusted random coefficient models (upper part of Table 3), persistent drinking, with and without persistent smoking, showed trends for higher FVC levels. As compared to never drinkers and never smokers, persistent drinkers who were also persistent smokers had a mean 128 ml increase in FVC (p=0.091) and persistent drinkers who never smoked a mean 121 ml increase in FVC (p=0.057). No significant FEV1 associations were found, whereas all drinking/smoking groups were found to have lower FEV1/FVC as compared to never drinkers and never smokers: −4.85% (p<0.001) for persistent drinkers who were also persistent smokers, −3.68% (p<0.001) for persistent drinkers who were never smokers, −5.19% (p<0.001) for persistent smokers who did not drink, and −2.04% (p=0.001) for inconsistent drinkers/smokers. Results from models including an interaction term with age (lower part of Table 3) showed that the effects of persistent drinking on FVC, with and without persistent smoking, were due to a significantly slower decline of FVC during adult life in both groups. Persistent drinkers who were also persistent smokers had a 5.8 ml/yr (p=0.045) slower FVC decline and persistent drinkers who never smoked a 6.1 ml/yr (p=0.010) slower decline. In contrast, the decline of FEV1/FVC was significantly faster among persistent smokers, with or without persistent drinking.
Table 3.
Random Coefficient Model (RCM) results with persistent drinking and smoking categories (N=1,333 subjects with 10,264 total observations)
| FVC in ml | FEV1 in ml | FEV1/FVC in % | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Coefficient | SE | P value | Coefficient | SE | P value | Coefficient | SE | P value | |
| MAIN EFFECTS | |||||||||
| Persistent Drink & Smoke categories* | |||||||||
| Non Drinker & Non Smoker (ref) | |||||||||
| Only Persistent Drink | 121.04 | 63.65 | 0.057 | −15.30 | 55.75 | 0.784 | −3.68 | 0.78 | <0.001 |
| Only Persistent Smoke | 65.79 | 119.84 | 0.583 | −140.43 | 104.73 | 0.180 | −5.19 | 1.47 | <0.001 |
| Persistent Drink & Smoke | 127.51 | 75.51 | 0.091 | −88.41 | 65.82 | 0.179 | −4.85 | 0.94 | <0.001 |
| Other (Not Persistent) | 52.63 | 51.94 | 0.311 | −15.62 | 44.90 | 0.728 | −2.04 | 0.64 | 0.001 |
| MAIN EFFECTS + INTERACTIONS ** | |||||||||
| Persistent Drink & Smoke categories* | |||||||||
| Non Drinker & Non Smoker (ref) | |||||||||
| Only Persistent Drink | −27.48 | 84.83 | 0.746 | −76.26 | 74.83 | 0.308 | −2.67 | 1.23 | 0.031 |
| Only Persistent Smoke | 61.61 | 165.43 | 0.710 | −2.35 | 144.11 | 0.987 | −0.27 | 2.41 | 0.910 |
| Persistent Drink & Smoke | −17.16 | 99.81 | 0.863 | 0.41 | 87.76 | 0.996 | −1.14 | 1.46 | 0.432 |
| Other (Not Persistent) | −47.93 | 70.98 | 0.499 | −12.13 | 61.46 | 0.844 | 0.22 | 1.04 | 0.829 |
| Only Persistent Drink X Age | 6.05 | 2.34 | 0.010 | 2.81 | 1.99 | 0.158 | −0.02 | 0.04 | 0.528 |
| Only Persistent Smoke X Age | −0.19 | 4.76 | 0.969 | −6.04 | 3.99 | 0.131 | −0.19 | 0.07 | 0.009 |
| Persistent Drink & Smoke X Age | 5.82 | 2.90 | 0.045 | −4.19 | 2.44 | 0.086 | −0.15 | 0.04 | 0.001 |
| Other (Not Persistent) X Age | 3.69 | 1.82 | 0.043 | −0.39 | 1.56 | 0.802 | −0.08 | 0.03 | 0.003 |
Adjusted for sex, age, height, pack-years, BMI categories, education categories
Age was centered at 21 years, so in these models main effects refer to associations of drinking and/or smoking categories with lung function at age 21 years
The relation of quantitative drinking exposure to lung function
When we analyzed data on quantitative drinking exposure, we found a significant trend for increase in FVC with increasing category of number of drinks per month (Table 4) (p for overall trend = 0.004). As compared to never drinkers, on average subjects who drank 15–30 drinks per month had an FVC increase of 36 ml, subjects who drank 30–90 drinks per month an FVC increase of 89 ml, and subjects who drank 90–140 drinks per month an FVC increase of 99 ml. The trend for FVC effects across the drinking exposure categories was significant (p=0.004). There was a border-line trend for a dose-response association with FEV1. In contrast, no significant dose-response association was found for number of drinks per month on FEV1/FVC levels.
Table 4.
Random Coefficient Model (RCM) results* with longitudinal drinking exposure categories (N=1,332 with 3,734 observations**)
| FVC in ml | FEV1 in ml | FEV1/FVC in % | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Coefficient | SE | P value | Coefficient | SE | P value | Coefficient | SE | P value |
| Drinking Exposure Categories (drinks per month) | |||||||||
| None (ref) | |||||||||
| <5 | 7.42 | 21.64 | 0.732 | 2.37 | 15.69 | 0.880 | −0.17 | 0.33 | 0.604 |
| 5–15 | −0.68 | 23.51 | 0.977 | 8.44 | 17.12 | 0.662 | 0.07 | 0.36 | 0.843 |
| 15–30 | 35.84 | 24.62 | 0.145 | 21.65 | 18.16 | 0.233 | −0.36 | 0.37 | 0.329 |
| 30–90 | 89.20 | 27.63 | 0.001 | 32.56 | 20.47 | 0.112 | −0.86 | 0.41 | 0.038 |
| 90–140 | 99.35 | 68.43 | 0.147 | 71.23 | 49.69 | 0.152 | 0.39 | 1.03 | 0.704 |
| Trend of Drinking Exposure Categories | 18.39 | 6.31 | 0.004 | 8.48 | 4.72 | 0.072 | −0.15 | 0.09 | 0.120 |
Adjusted for sex, age, height, smoking category, pack-years, BMI categories, education categories
These analyses included only lung function data from the same four surveys when alcohol intake was measured
The relation of persistent alcohol intake to spirometric patterns
The percent of participants within each drinking category that, at any point during the study, had spirometric restriction, obstruction, or both was respectively 14.2%, 17.5%, 17.2% among never drinkers, 9.7%, 23.9%, 13.9% among intermittent drinkers, and 6.0%, 22.1%, and 10.0% among persistent drinkers. When tested in multinomial logistic regression models, we found that, after adjustment for sex, age, education, BMI categories, smoking status, and pack-years, persistent drinkers were less likely to have spirometric restriction than never drinkers (adjusted Relative Risk Ratio: 0.52, p=0.003), with inconsistent drinkers showing similar results (adjRRR: 0.66, p=0.043). No significant effects on spirometric obstruction were found for persistent and inconsistent drinking (RRRs: 1.23, p=0.221; and 1.31, p=0.123; respectively).
DISCUSSION
In this study we found persistent light-to-moderate drinkers to have a slower decline of FVC with age, which resulted in higher FVC values in later life and lower risk of spirometric restriction. Our statistical models suggested that FVC levels were comparable between drinkers and non-drinkers at young adult life, and that the overall FVC differences between these two groups were due to different rates of FVC decline over the adult life. Thus, even though the average effect of alcohol intake on FVC decline appeared of relatively modest magnitude, these effects may become clinically relevant towards late adult life when they had multiple decades to accumulate over time. FEV1 showed a similar pattern, but of smaller magnitude so that FEV1/FVC ratios were in fact lower in drinkers. These associations showed a dose-response relationship, which persisted after adjustment for potential confounders, including smoking status and pack-years.
Our results show increased FVC levels in light to moderate drinkers and therefore they should not be considered in contrast with previous cross-sectional studies that have shown negative associations of alcohol with lung function parameters and/or respiratory symptoms among heavy drinkers (Emirgil & Sobol, 1977; Emirgil, Sobol, Heymann, & Shibutani, 1974; Frantz et al., 2014; Garshick et al., 1989; Lyons, Howard, Milledge, & Peters, 1986). These studies specifically recruited subjects from alcohol detoxification units, rehabilitation units, or those who were referred for assessment or treatment of alcohol use disorders, or they selected participants with extreme alcohol intake from population-based samples. Indeed, a previous study from the same TESAOD cohort that used baseline alcohol and lung function parameters found heavy drinking to be associated with respiratory symptoms, particularly in association with heavy smoking (Lebowitz, 1981). In contrast, studies that included subjects with varied levels of alcohol intake have provided different, and somewhat inconsistent, results with some showing no association of alcohol with lung function after accounting for smoking status (Cohen et al., 1980; Sparrow et al., 1983), others showing negative associations (Lange et al., 1988; Zureik et al., 1996), but the majority showing enhanced lung function among light to moderate drinkers (Root et al., 2014; Schunemann et al., 2002; Sisson et al., 2005; Siu et al., 2010; Tabak, Smit, Heederik, et al., 2001; Tabak, Smit, Rasanen, et al., 2001; Twisk et al., 1998). These inconsistencies may be related to large inter-study differences in alcohol intake, age distributions, study design, and heterogeneity in other relevant demographic, clinical, and behavioral factors. In this framework it should be noted that in our study, while alcohol intake was associated with higher FVC levels, its association with FEV1/FVC levels was of opposite direction. Therefore, our findings suggest that from a clinical standpoint alcohol intake may have differential effects on spirometric restriction and obstruction, being associated with protection from the former but with increased risk for the latter. Previous studies have consistently shown that both spirometric restriction(Godfrey & Jankowich, 2016; Guerra et al., 2010) and obstruction (Vestbo et al., 2013) carry a significant morbidity and mortality burden at the population level. However, larger studies will be required to determine the clinical relevance of alcohol intake in these scenarios.
Various mechanisms may explain the association that we found between light to moderate alcohol intake and higher FVC values. First, while heavy alcohol exposure may impair mucociliary clearance, exposure to light to moderate alcohol may actually improve airway clearance. A study looking at particle clearance rates following alcohol ingestion showed faster clearance rates in moderate drinkers (Venizelos, Gerrity, & Yeates, 1981), although there is no definitive evidence in human studies of the direct effect of alcohol on mucociliary clearance in the current literature (Sisson, 2007). Second, alcohol has been shown to act as a bronchodilator in patients with asthma when ingested orally (J. Ayres, Ancic, & Clark, 1982) or taken intravenously (J. G. Ayres & Clark, 1983), even though it may provoke bronchospasm when inhaled (Hooper et al., 1995; Zuskin, Bouhuys, & Saric, 1981). However, in our study we found the strongest effects of alcohol intake on FVC, not FEV1. Third, moderate intake of alcohol may reduce systemic inflammation, a known risk factor for impaired lung function. A previous study showed that markers of systemic inflammation, such as white blood counts, C-reactive protein levels, and fibrinogen levels were reduced with alcohol intake (Sisson et al., 2005). Lastly, alcohol may affect other factors that are, in turn, associated with FVC and spirometric restriction. For example, obesity is a known risk factor for lung restriction and heavy drinkers may metabolize their calories differently and be less likely to be obese (Addolorato, Capristo, Greco, Stefanini, & Gasbarrini, 1998).
A limitation of our study is the potential for inaccurate reporting of alcohol consumption. Information on alcohol intake was collected at four surveys (1, 7, 11, and 12). Given the missing availability of drinking data between surveys, there is potential for the misclassification of subjects into their respective longitudinal drinking categories. However, this misclassification is likely to be non-differential (i.e., to apply to participants independent of their lung function) and, therefore, its most likely consequence is bias towards the null hypothesis. In explaining our results, the possibilities of residual confounding and reverse causality should be acknowledged. In relation to the former, important differences in basic characteristics were noted across drinking categories, with persistent drinkers being more likely to be young, males, and smokers with higher educational attainment than never drinkers. However, it is noteworthy that all models were adjusted for these covariates. As for the possibility of reverse causality, it is possible that healthy subjects are more likely to drink, and our non-drinking and inconsistent drinking categories may have included subjects who did not drink or quit drinking due to illness (Shaper, Wannamethee, & Walker, 1988). Although our findings showed longitudinal effects of drinking on decline of lung function and a dose-response relationship, we acknowledge that our observational data cannot completely rule out reverse causality. Furthermore, the study population only included non-Hispanic white subjects and therefore results may not be generalizable to other races or ethnicities.
Among the strengths of this study is our large sample size of subjects with available drinking information over a 20 year follow up period. Also, these subjects performed repeated pulmonary function tests in up to 11 surveys which allows for the comprehensive assessment of lung function trajectory over time. Given the known and potentially harmful effects of heavy alcohol consumption, we restricted analysis to light and moderate consumers of alcohol and, in turn, removed the influence of heavy drinking. Although main results were confirmed in sensitivity analyses that also included participants who reported more than 140 drinks per month, the number of these heavier drinkers was too small in our study to determine conclusively whether they differed from lighter drinkers.
In conclusion, in a long-term population-based cohort, after taking into account the potential confounding effects of smoking, we found persistent light to moderate alcohol consumption to decrease significantly the rate of FVC decline over adult life and to be associated with protection from spirometric restriction. The nature of these associations warrants further scrutiny.
Supplementary Material
Table E1. Random Coefficient Model (RCM) results with persistent drinking categories, excluding heavy drinkers defined as subjects who at any survey reported drinking more than 90 drinks per month (N=1,297 subjects with 9,994 total observations)
Table E2. Random Coefficient Model (RCM) results with persistent drinking and smoking categories, excluding heavy drinkers defined as subjects who at any survey reported drinking more than 90 drinks per month (N=1,297 subjects with 9,994 total observations)
Table E3. Random Coefficient Model (RCM) results* with longitudinal drinking exposure categories, excluding heavy drinkers defined as subjects who at any survey reported drinking more than 90 drinks per month (N=1,296 with 3,636 observations **)
Table E4. Random Coefficient Model (RCM) results with persistent drinking categories, including heavy drinkers (N=1,376 subjects with 10,623 total observations)
Table E5. Random Coefficient Model (RCM) results with persistent drinking and smoking categories, including heavy drinkers (N=1,376 subjects with 10,623 total observations)
Table E6. Random Coefficient Model (RCM) results* with longitudinal drinking exposure categories, including heavy drinkers (N=1,375 with 3,867 observations **)
Table E7. Characteristics of subjects across drinking groups and surveys
Figure E1. Predicted levels and decline of FEV1 from age 21 years onward across persistent drinking categories in TESAOD
Lines represent predicted values across the drinking categories for a non smoking female, with a height of 168 cm, normal body mass index, and more than a high school education.
Figure E2. Predicted levels and decline of FEV1/FVC from age 21 years onward across persistent drinking categories in TESAOD
Lines represent predicted values across the drinking categories for a non smoking female, with a height of 168 cm, normal body mass index, and more than a high school education.
Highlights.
Effects of persistent alcohol intake on trajectories of lung function are unknown.
Persistent light-to-moderate drinkers had a slower decline of FVC with age.
Effects persisted after adjustment for confounders including smoking.
Acknowledgments
Funding: This work was supported by awards HL107188 and HL095021 from the National Heart, Lung, and Blood Institute and award R01 AA008769-24 from the National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Addolorato G, Capristo E, Greco AV, Stefanini GF, Gasbarrini G. Influence of chronic alcohol abuse on body weight and energy metabolism: is excess ethanol consumption a risk factor for obesity or malnutrition? J Intern Med. 1998;244(5):387–395. doi: 10.1046/j.1365-2796.1998.00381.x. [DOI] [PubMed] [Google Scholar]
- Ayres J, Ancic P, Clark TJ. Airways responses to oral ethanol in normal subjects and in patients with asthma. J R Soc Med. 1982;75(9):699–704. doi: 10.1177/014107688207500905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayres JG, Clark TJ. Intravenous ethanol can provide bronchodilatation in asthma. Clin Sci (Lond) 1983;64(5):555–557. doi: 10.1042/cs0640555. [DOI] [PubMed] [Google Scholar]
- Brown H, Prescott R. Applied mixed models in medicine. Chichester, UK: John Wiley & Sons, LTD; 2001. [Google Scholar]
- Cohen BH, Celentano DD, Chase GA, Diamond EL, Graves CG, Levy DA, … Tockman MS. Alcohol consumption and airway obstruction. Am Rev Respir Dis. 1980;121(2):205–215. doi: 10.1164/arrd.1980.121.2.205. [DOI] [PubMed] [Google Scholar]
- Emirgil C, Sobol BJ. Pulmonary function in former alcoholics. Chest. 1977;72(1):45–51. doi: 10.1378/chest.72.1.45. [DOI] [PubMed] [Google Scholar]
- Emirgil C, Sobol BJ, Heymann B, Shibutani K. Pulmonary function in alcoholics. Am J Med. 1974;57(1):69–77. doi: 10.1016/0002-9343(74)90770-0. [DOI] [PubMed] [Google Scholar]
- Frantz S, Wollmer P, Dencker M, Engstrom G, Nihlen U. Associations between lung function and alcohol consumption--assessed by both a questionnaire and a blood marker. Respir Med. 2014;108(1):114–121. doi: 10.1016/j.rmed.2013.08.041. [DOI] [PubMed] [Google Scholar]
- Garshick E, Segal MR, Worobec TG, Salekin CM, Miller MJ. Alcohol consumption and chronic obstructive pulmonary disease. Am Rev Respir Dis. 1989;140(2):373–378. doi: 10.1164/ajrccm/140.2.373. [DOI] [PubMed] [Google Scholar]
- Godfrey MS, Jankowich MD. The Vital Capacity Is Vital: Epidemiology and Clinical Significance of the Restrictive Spirometry Pattern. Chest. 2016;149(1):238–251. doi: 10.1378/chest.15-1045. [DOI] [PubMed] [Google Scholar]
- Guerra S, Sherrill DL, Venker C, Ceccato CM, Halonen M, Martinez FD. Morbidity and mortality associated with the restrictive spirometric pattern: a longitudinal study. Thorax. 2010;65(6):499–504. doi: 10.1136/thx.2009.126052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper G, Steed KP, Gittins DP, Newman SP, Richards A, Rubin I. Bronchoconstriction following inhaled ethanol solutions. Respir Med. 1995;89(6):457–458. doi: 10.1016/0954-6111(95)90221-x. [DOI] [PubMed] [Google Scholar]
- Knudson RJ, Slatin RC, Lebowitz MD, Burrows B. The maximal expiratory flow-volume curve. Normal standards, variability, and effects of age. Am Rev Respir Dis. 1976;113(5):587–600. doi: 10.1164/arrd.1976.113.5.587. [DOI] [PubMed] [Google Scholar]
- Lange P, Groth S, Mortensen J, Appleyard M, Nyboe J, Jensen G, Schnohr P. Pulmonary function is influenced by heavy alcohol consumption. Am Rev Respir Dis. 1988;137(5):1119–1123. doi: 10.1164/ajrccm/137.5.1119. [DOI] [PubMed] [Google Scholar]
- Lebowitz MD. Respiratory symptoms and disease related to alcohol consumption. Am Rev Respir Dis. 1981;123(1):16–19. doi: 10.1164/arrd.1981.123.1.16. [DOI] [PubMed] [Google Scholar]
- Lebowitz MD, Knudson RJ, Burrows B. Tucson epidemiologic study of obstructive lung diseases. I: Methodology and prevalence of disease. Am J Epidemiol. 1975;102(2):137–152. doi: 10.1093/oxfordjournals.aje.a112141. [DOI] [PubMed] [Google Scholar]
- Lyons DJ, Howard SV, Milledge JS, Peters TJ. Contribution of ethanol and cigarette smoking to pulmonary dysfunction in chronic alcoholics. Thorax. 1986;41(3):197–202. doi: 10.1136/thx.41.3.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, … Wanger J. Interpretative strategies for lung function tests. Eur Respir J. 2005;26(5):948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- Root MM, Houser SM, Anderson JJ, Dawson HR. Healthy Eating Index 2005 and selected macronutrients are correlated with improved lung function in humans. Nutr Res. 2014;34(4):277–284. doi: 10.1016/j.nutres.2014.02.008. [DOI] [PubMed] [Google Scholar]
- Schunemann HJ, Grant BJ, Freudenheim JL, Muti P, McCann SE, Kudalkar D, … Trevisan M. Evidence for a positive association between pulmonary function and wine intake in a population-based study. Sleep Breath. 2002;6(4):161–173. doi: 10.1007/s11325-002-0161-6. [DOI] [PubMed] [Google Scholar]
- Shaper AG, Wannamethee G, Walker M. Alcohol and mortality in British men: explaining the U-shaped curve. Lancet. 1988;2(8623):1267–1273. doi: 10.1016/s0140-6736(88)92890-5. [DOI] [PubMed] [Google Scholar]
- Sisson JH. Alcohol and airways function in health and disease. Alcohol. 2007;41(5):293–307. doi: 10.1016/j.alcohol.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisson JH, Stoner JA, Romberger DJ, Spurzem JR, Wyatt TA, Owens-Ream J, Mannino DM. Alcohol intake is associated with altered pulmonary function. Alcohol. 2005;36(1):19–30. doi: 10.1016/j.alcohol.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Siu ST, Udaltsova N, Iribarren C, Klatsky AL. Alcohol and lung airways function. Perm J. 2010;14(1):11–18. doi: 10.7812/tpp/09-089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow D, Rosner B, Cohen M, Weiss ST. Alcohol consumption and pulmonary function. A cross-sectional and longitudinal study. Am Rev Respir Dis. 1983;127(6):735–738. doi: 10.1164/arrd.1983.127.6.735. [DOI] [PubMed] [Google Scholar]
- Tabak C, Smit HA, Heederik D, Ocke MC, Kromhout D. Diet and chronic obstructive pulmonary disease: independent beneficial effects of fruits, whole grains, and alcohol (the MORGEN study) Clin Exp Allergy. 2001;31(5):747–755. doi: 10.1046/j.1365-2222.2001.01064.x. [DOI] [PubMed] [Google Scholar]
- Tabak C, Smit HA, Rasanen L, Fidanza F, Menotti A, Nissinen A, … Kromhout D. Alcohol consumption in relation to 20-year COPD mortality and pulmonary function in middle-aged men from three European countries. Epidemiology. 2001;12(2):239–245. doi: 10.1097/00001648-200103000-00018. [DOI] [PubMed] [Google Scholar]
- Twisk JW, Staal BJ, Brinkman MN, Kemper HC, van Mechelen W. Tracking of lung function parameters and the longitudinal relationship with lifestyle. Eur Respir J. 1998;12(3):627–634. doi: 10.1183/09031936.98.12030627. [DOI] [PubMed] [Google Scholar]
- Venizelos PC, Gerrity TR, Yeates DB. Response of human mucociliary clearance to acute alcohol administration. Arch Environ Health. 1981;36(4):194–201. doi: 10.1080/00039896.1981.10667625. [DOI] [PubMed] [Google Scholar]
- Vestbo J, Hurd SS, Agusti AG, Jones PW, Vogelmeier C, Anzueto A, … Rodriguez-Roisin R. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347–365. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- Zureik M, Liard R, Kauffmann F, Henry C, Neukirch F. Alcohol consumption, gamma-glutamyl transpeptidase (GGT), and pulmonary function: a cross-sectional and longitudinal study in working men. Alcohol Clin Exp Res. 1996;20(9):1507–1511. doi: 10.1111/j.1530-0277.1996.tb01691.x. [DOI] [PubMed] [Google Scholar]
- Zuskin E, Bouhuys A, Saric M. Lung function changes by ethanol inhalation. Clin Allergy. 1981;11(3):243–248. doi: 10.1111/j.1365-2222.1981.tb01590.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table E1. Random Coefficient Model (RCM) results with persistent drinking categories, excluding heavy drinkers defined as subjects who at any survey reported drinking more than 90 drinks per month (N=1,297 subjects with 9,994 total observations)
Table E2. Random Coefficient Model (RCM) results with persistent drinking and smoking categories, excluding heavy drinkers defined as subjects who at any survey reported drinking more than 90 drinks per month (N=1,297 subjects with 9,994 total observations)
Table E3. Random Coefficient Model (RCM) results* with longitudinal drinking exposure categories, excluding heavy drinkers defined as subjects who at any survey reported drinking more than 90 drinks per month (N=1,296 with 3,636 observations **)
Table E4. Random Coefficient Model (RCM) results with persistent drinking categories, including heavy drinkers (N=1,376 subjects with 10,623 total observations)
Table E5. Random Coefficient Model (RCM) results with persistent drinking and smoking categories, including heavy drinkers (N=1,376 subjects with 10,623 total observations)
Table E6. Random Coefficient Model (RCM) results* with longitudinal drinking exposure categories, including heavy drinkers (N=1,375 with 3,867 observations **)
Table E7. Characteristics of subjects across drinking groups and surveys
Figure E1. Predicted levels and decline of FEV1 from age 21 years onward across persistent drinking categories in TESAOD
Lines represent predicted values across the drinking categories for a non smoking female, with a height of 168 cm, normal body mass index, and more than a high school education.
Figure E2. Predicted levels and decline of FEV1/FVC from age 21 years onward across persistent drinking categories in TESAOD
Lines represent predicted values across the drinking categories for a non smoking female, with a height of 168 cm, normal body mass index, and more than a high school education.