Figure 1. Human VWF variant expression and function.
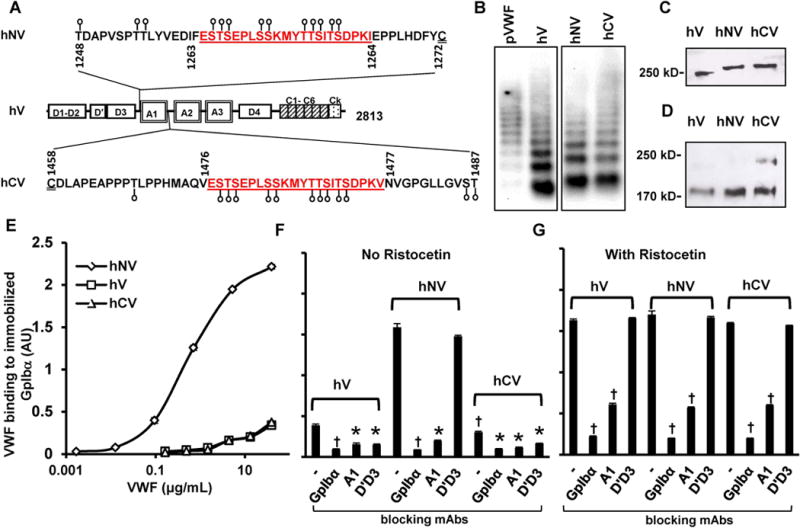
A. The A1-domain of wild-type human VWF (‘hV’) is flanked by a few potential O-linked glycosylation sites at both the N- (aa 1248-1271) and C-terminus (1459-1487). These O-glycans are depicted by lollipop symbols. A 22 amino acid mucin-repeat motif from human CD43 (underlined) replaced Ser1263 to generate the VWF-variant ‘hNV’. Insertion of the same 22 aa sequence at the C-terminus in place of Thr1477 resulted in ‘hCV’. B. Agarose gel electrophoresis-western blotting of non-reduced VWF variants expressed in HEK293T cells. pVWF is human plasma VWF from humate-P. C. 8% SDS-PAGE analysis of VWF variants showing the higher molecular mass of hNV and hCV. D. hCV cleavage by ADAMTS13 is partly reduced compared to other proteins after 24h digestion. E. Binding of different concentrations of VWF variants to 4 μg/mL immobilized GpIbα-Fc in microtiter plates. hNV binding is remarkably higher. F-G. Binding of VWF variants to immobilized GpIbα-Fc in the absence (F) or presence of ristocetin (G). 20 μg/mL of indicated blocking mAbs was added in some cases: anti-GpIbα mAb AK2; anti-VWF A1-domain clone AVW-3; and anti-VWF D’D3 mAb DD3.1. All ELISA data are from 2-4 independent runs, each with 2-3 repeats. Error bars are too small to be visible in some cases. †P<0.05 with respect to all other treatments. *P<0.05 with respect to all other treatments except that treatments indicated by * are not different from each other.
