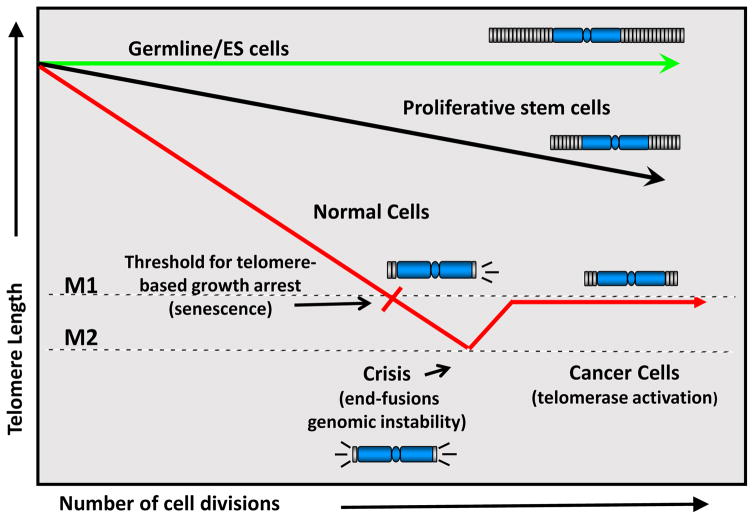
Figure 2.
Germline and embryonic stem (ES) cells retain full or almost full telomere length due to expression of telomerase activity. Pluripotent proliferative stem cells have regulated telomerase activity and thus they lose telomeres throughout life but at a reduced rate. Most somatic cells do not express telomerase activity and thus lose telomere length with each division at a faster rate compared to stem cells until the cells uncap a few of their telomeres and undergo a growth arrest called replicative senescence (or M1, Mortality Stage 1). In the absence of cell cycle checkpoints (e.g. p53/pRB pathway), cells bypass senescence until they reach crisis (or M2, Mortality Stage 2). In crisis, telomeres are so short that chromosome end fusions occur and there is increased genomic instability (probably due to chromosomal, breakage-fusion- bridge cycles). A rare cell that escapes crisis almost universally does so by reactivating telomerase and this cell can now become a cancer cell with limitless potential to divide. When telomerase is activated in crisis, most cancer cells have short telomeres and just the right amount of telomerase to maintain the shortest telomeres. However, in certain types of cancer such as malignant melanoma some invasive tumors have very long telomeres but lack telomerase or an ALT mechanism to maintain the telomeres (as illustrated in Shay, 2017). In this case, it is possible that when cells escape from crisis, there is a large amount of telomerase produced, telomeres grow substantially, and due to telomere looping the TERT locus is again silenced. These tumors can be aggressive since they have very long telomeres but they would still progressively shorten their telomeres with each cell division (Shay, 2017).