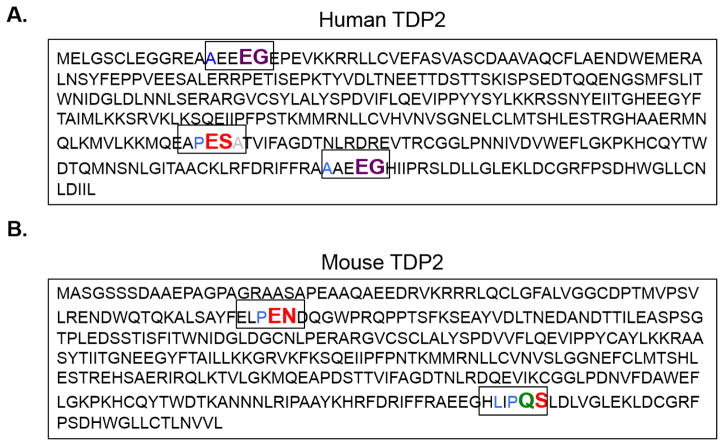
Fig. 4. Putative picornavirus 3C and 3CD proteinase cleavage sites in the TDP2 sequence.
The picornavirus 3C proteinase and its precursor 3CD recognize specific residues at putative cleavage sites. Enterovirus 3C/3CD recognize QG, QA, QN, and QS residues with an amino acid with an aliphatic side chain in the P4 position (cleavage site colored in green). Rhinovirus 3C/3CD recognizes the same cleavage sites as the enterovirus cleavage sites as well as an additional site: EG, also with an aliphatic side chain in the P4 position (cleavage site colored in purple). Cardiovirus 3C proteinases recognize QG, QA, QS, EN and ES residues with a proline preferred in the P2 or P2′ position (cleavage sites colored in red). Putative cleavage sites are highlighted in their denoted color in both the (A) human and (B) mouse TDP2 sequences.