Abstract
Pancreaticoduodenal (PD) artery aneurysms account for less than 2% of all splanchnic aneurysms. A mycotic aetiology is extremely uncommon.
Two weeks following an episode of sepsis related to a prostatic biopsy, a 59-year-old man presented with abdominal pain and anaemia. Ultrasonography and computed tomography revealed an inferior PD artery pseudoaneurysm with an associated mesenteric root haematoma. This was treated successfully by transcatheter embolisation.
Infective pseudoaneurysms of the PD artery are rare but can be associated with rupture into the gastrointestinal tract or retroperitoneum. Transcatheter embolisation remains the most effective therapy as it is associated with low morbidity and mortality rates and recurrence is very unlikely provided the aneurysm is completely excluded from the circulation.
Keywords: Infective aneurysm, Visceral aneurysm, Inferior pancreaticoduodenal artery, Angiogram, Coil embolization
Aneurysmal disease of the pancreaticoduodenal (PD) arteries is rare, accounting for less than 2% of all splanchnic aneurysms.1,2 Over 50% of cases are true aneurysms due to arteriosclerosis.1–3 The remainder are pseudoaneurysms, most commonly related to pancreatitis. Other causes include trauma, previous surgery, penetrating duodenal ulcers, infection, carcinomas and arteritis.1–3
Case history
A 59-year-old man developed prostatitis with severe systemic sepsis three days after a prostatic biopsy and despite prophylactic ciprofloxacin (400mg on induction of anaesthesia). He presented with dysuria, haematuria, fever and rigors and was treated with intravenous co-amoxiclav (1.2g three times daily) and gentamicin (240mg intravenously once daily) for three days. Although his urine was positive for nitrites, suggesting a urinary tract infection, no organisms were identified. The patient’s symptoms resolved and he was discharged.
However, two weeks later he re-presented with diarrhoea, abdominal pain and distension; the diarrhoea was not associated with blood or mucus. His past medical history included type 2 diabetes mellitus, hypertension and hypercholesterolaemia. An abdominal examination revealed some tenderness in the periumbilical region and right upper quadrant but no peritonitis. He was mildly anaemic (haemoglobin: 12.5gm/dl; normal: 13–17gm/dl) and had an elevated C-reactive protein level (46mg/l; normal: <5mg/l). A plain abdominal radiograph was unremarkable. The symptoms improved after empirical treatment with metronidazole for presumed Clostridium difficile. A barium enema and ultrasonography were requested prior to outpatient review.
Abdominal ultrasonography 10 days later detected a well defined mass measuring 10cm x 2cm related to the right side of the proximal superior mesenteric artery, with no aortic communication. The appearance suggested a mesenteric haematoma. Urgent computed tomography confirmed the presence of a mesenteric haematoma intimately related to a small, focal, enhancing aneurysm of the inferior PD artery (Fig 1). In addition, the coeliac axis origin was compressed by the median arcuate ligament. There was no free fluid within the abdominal cavity.
Figure 1.
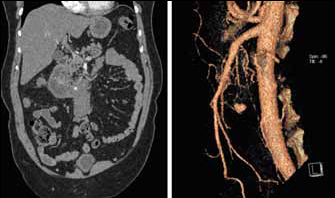
Coronal reformatted image from contrast enhanced computed tomography demonstrating small pseudoaneurysm related to the superior aspect of a mesenteric root haematoma (left); 3D reconstruction showing pseudoaneurysm of inferior pancreaticoduodenal artery (right)
A visceral arteriogram demonstrated a small pseudoaneurysm of the main trunk of the anterior inferior PD artery, which did not involve a bifurcation. There was mild hypertrophy of the PD arcade related to the coeliac axis compression. The anterior inferior PD was embolised with platinum microcoils on either side of the aneurysmal cavity. Final angiography confirmed complete exclusion of the aneurysm from the circulation, with good preservation of the other PD arcade branches (Fig 2).
Figure 2.
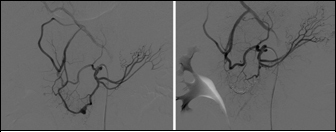
Selective inferior pancreaticoduodenal (PD) artery angiogram before and after embolisation demonstrating the focal anterior inferior PD artery pseudoaneurysm (left) and its subsequent exclusion from the circulation by platinum microcoils with preservation of the posterior inferior PD artery (right)
The diagnosis of infective pseudoaneurysm was based on the temporal relationship between the episode of severe septicaemia (although no causative agent was identified) and the spontaneous haemorrhage into the root of the mesentery, plus the angiographic appearances of the pseudoaneurysm, which did not involve a vessel bifurcation.
Discussion
Aneurysmal disease of the PD arteries is rare, with approximately 100 cases reported in the literature to date. These aneurysms account for 2% of all splanchnic artery aneurysms.1–3 Just over half are true arteriosclerotic aneurysms and many of these are associated with median arcuate ligament compression of the coeliac axis.1,2,4–7 The high blood flow through the PD arcade, from the superior mesenteric artery to the common hepatic artery, causes turbulence leading to aneurysm formation.1,4,6,8 The PD arcade appears particularly susceptible to this aneurysmal degeneration, which tends to occur at vessel bifurcations.4 Although very uncommon, rupture is usually retroperitoneal.5 Median arcuate ligament compression was seen in the present case but there was only minor hypertrophy of the PD arcade and the aneurysmal disease did not involve a vessel bifurcation. The compression is therefore unlikely to have been more than a minor contributory factor.
Pancreatitis, either acute or chronic, accounts for 30% of all PD pseudoaneurysms;1,4,5 presumably, the inflammatory process weakens the arterial wall.1,4 These false aneurysms often rupture into the gastrointestinal tract,4,5 potentially via the pancreatic duct (haemosuccus pancreaticus). There were no features of pancreatitis in the present patient. Other causes of PD pseudoaneurysms include trauma, previous surgery, penetrating duodenal ulcers, carcinomas and arteritis.1,4 Infective pseudoaneurysms are rare but the close relationship with post-prostatic biopsy sepsis and pseudoaneurysm supports this aetiological mechanism in the present case.
Endovascular therapy is replacing operative ligation in the management of PD aneurysms.1–3,5 Transcatheter embolisation is minimally invasive with a low risk of either serious complications or recurrent bleeding (when correctly performed).3,5,6 The mortality rate of embolisation is zero, as opposed to 19% in surgical series.3,5,6 Coaxial catheters are frequently required because of the small calibre and tortuosity of the vessels.3,5 Successful embolisation entails isolating the pseudoaneurysm from the circulation by placing embolic material on either side of its neck.1,3,9 Metallic coils are generally used, either alone or in combination with Amplatzer® vascular plugs and n-butyl-2-cyanoacrylate.5 Rarely, rupture of the aneurysm during embolisation occurs at the time of vessel occlusion and is controlled by completing the procedure.9 Operative intervention is sometimes still needed when selective catheterisation cannot be achieved2 or reconstruction of the coeliac axis is required to prevent subsequent mesenteric ischaemia.6
References
- 1.Suzuki K, Tachi Y, Ito S et al. Endovascular management of ruptured pancreaticoduodenal artery aneurysms associated with celiac axis stenosis. Cardiovasc Intervent Radiol 2008; 31: 1,082–1,087. [DOI] [PubMed] [Google Scholar]
- 2.Jibiki M, Inoue Y, Iwai T et al. Treatment of three pancreaticoduodenal artery aneurysms associated with coeliac artery occlusion and splenic artery aneurysm: a case report and review of the literature. Eur J Vasc Endovasc Surg 2005; 29: 213–217. [DOI] [PubMed] [Google Scholar]
- 3.Ikeda O, Tamura Y, Nakasone Y et al. Nonoperative management of unruptured visceral artery aneurysms: treatment by transcatheter coil embolization. J Vasc Surg 2008; 47: 1,212–1,219. [DOI] [PubMed] [Google Scholar]
- 4.Chiang KS, Johnson CM, McKusick MA et al. Management of inferior pancreaticoduodenal artery aneurysms: a 4-year, single center experience. Cardiovasc Intervent Radiol 1994; 17: 217–221. [DOI] [PubMed] [Google Scholar]
- 5.Murata S, Tajima H, Fukunaga T et al. Management of pancraticoduodenal artery aneurysms: results of superselective transcatheter embolization. Am J Roentgenol 2006; 187: W290–W298. [DOI] [PubMed] [Google Scholar]
- 6.Golarz SR, Hohmann S. Obstruction of the celiac axis resulting in a pancreaticoduodenal artery aneurysm. Proc (Bayl Univ Med Cent) 2009; 22: 330–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bellosta R, Luzzani L, Carugati C et al. Pancreaticoduodenal artery aneurysms associated with celiac axis occlusion. Ann Vasc Surg 2005; 19: 534–539. [DOI] [PubMed] [Google Scholar]
- 8.Sutton D, Lawton G. Coeliac stenosis or occlusion with aneurysm of the collateral supply. Clin Radiol 1973; 24: 49–53. [DOI] [PubMed] [Google Scholar]
- 9.Jackson JE. Management of Visceral Aneurysms. In: Mauro MA, Murphy K, Thomson K et al., eds. Image-Guided Interventions. Philadelphia, PA: Saunders; 2008. pp851–862. [Google Scholar]


