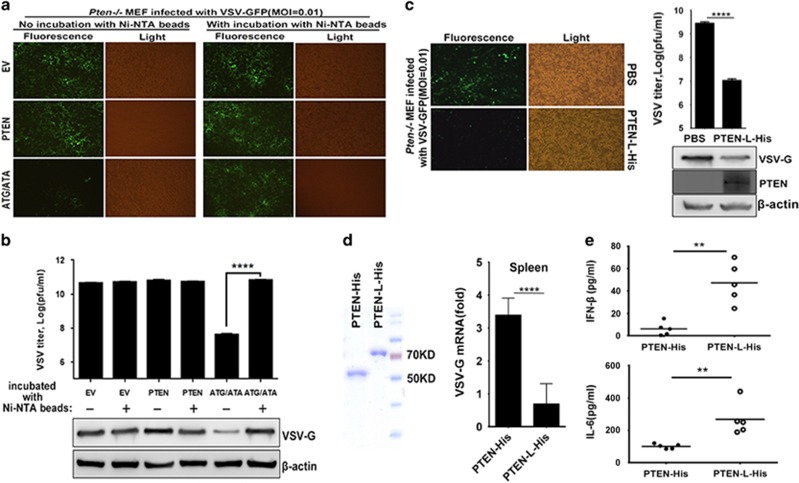
Figure 5.
Extracellular application of PTEN-L protein mediates antiviral responses in cell cultures and in vivo. (a) The supernatants of indicated transfected HEK293T cells were incubated with or without Ni-NTA magnetic agarose beads and used to treat Pten−/− MEF. VSV-GFP (MOI=0.01) was used to infect the treated Pten−/− MEF, and VSV-GFP replication was observed by microscopy. (b) Supernatants were subjected to VSV plaque assays. Cells were lysed and then subjected to SDS-PAGE. VSV replication was analyzed by anti-VSV-G and β-actin immunoblotting. (c) Pten−/− MEF cells were treated with PBS or 100 nM purified proteins from pRSFDuet-1-PTEN-LΔN21-His (PTEN-L-His) and infected by VSV-GFP (MOI=0.01). Supernatants were subjected to VSV plaque assays. Cells were lysed and then subjected to SDS-PAGE. VSV replication was analyzed by anti-VSV-G and β-actin immunoblotting. PTEN-L-His penetration into Pten−/− MEF cells was confirmed by anti-PTEN immunoblotting. (d) Recombinant purified PTEN-His and PTEN-L-His used for mice experiments were analyzed by SDS-PAGE and Coomassie BB staining. PTEN mutant mice were injected with purified PTEN or PTEN-L protein (5 mg/kg) for three days and then infected intraperitoneally with VSV at 1 × 108 pfu per mouse (n⩾5; 6 weeks old). Quantitative RT-PCR analysis of mRNA encoding VSV-G protein in the spleen in the indicated treated mice after infected with VSV for 24 h. (e) ELISA analysis of IFN-β and IL-6 in serum from PTEN- or PTEN-L-treated mice was also performed. The data represent average of three independent experiments and were analyzed by two-tailed unpaired t test. Graphs indicate mean±s.d. (n=3) derived from three independent experiments. **P<0.01, ****P<0.0001.