Abstract
We report the case of a 71-year-old man with afferent loop obstruction (ALO) after Roux-en-Y reconstruction due to gastric cancer. Computed tomography showed a distended afferent loop and a dilatated bile duct. We could not reach the stricture site in the afferent loop using a gastroscope. We performed percutaneous transhepatic biliary drainage (PTBD) and placed a self-expanding metallic stent (SEMS) in the duodenal stricture through the PTBD route. Although an endoscopic approach is preferable, when PTBD can be performed, percutaneous transhepatic SEMS placement might be an alternative option for treating ALO in cases in which it is not possible to reach the site of stenosis with an endoscope.
Keywords: afferent loop obstruction, self-expanding metallic stent, percutaneous transhepatic biliary drainage
Introduction
Afferent loop obstruction (ALO) is a rare complication that occurs after gastrojejunostomy. In most cases, ALO develops in association with gastrectomy and Billroth II or Roux-en-Y reconstruction, or pancreaticoduodenectomy with a conventional loop or Roux-en-Y reconstruction (1). Various etiologies of ALO have been reported, and the treatment differs between benign and malignant etiologies. Surgery is appropriate for benign ALO, while less invasive, non-surgical therapy is preferred for malignant ALO because these patients are in poor condition due to the advanced stage of their malignancy. Since the development of through-the-scope (TTS)-type enteral self-expanding metallic stents (SEMSs) and the double-balloon enteroscope (DBE), the enteral placement of an SEMS at the stricture site has been reported to be an effective treatment for malignant ALO (2-5). However, DBEs are not available at all institutions, and the stricture site cannot be reached endoscopically in all cases of ALO. Thus, an alternative approach is required. The placement of a transhepatic enteral stent (for a stricture in the afferent loop) or direct percutaneous drainage of the afferent loop, are good alternative methods (6). To the best of our knowledge, there are eight case reports describing the placement of a transhepatic SEMS for the treatment of malignant ALO (6-12). We herein report a case of malignant ALO in which percutaneous transhepatic SEMS placement was useful.
Case Report
A 71-year-old man underwent subtotal gastrectomy with Roux-en-Y reconstruction and cholecystectomy for gastric cancer, which was diagnosed as moderately differentiated adenocarcinoma. Adjuvant chemotherapy with S-1 (tegafur-gimeracil-oteracil potassium) was administered after surgery, but he was scheduled to change to UFT (tegafur-uracil) due to an S-1 drug allergy. We changed the chemotherapy regimen from UFT to irinotecan/cisplatin, paclitaxel, and docetaxel due to the recurrence of peritoneal dissemination and liver metastasis. Two years after surgery, he was readmitted with epigastric pain, high fever, and jaundice.
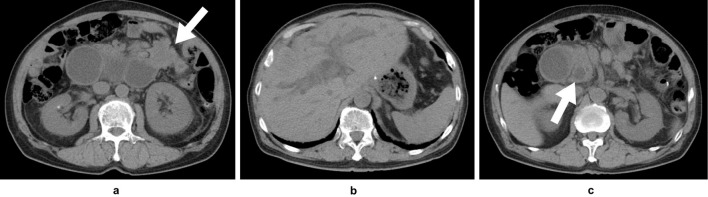
The laboratory data on readmission showed elevated serum levels of aspartate aminotransferase (138 U/L; normal range: 13-33 U/L), alanine aminotransferase (114 U/L; normal range: 6-30 U/L), alkaline phosphatase (3,419 U/L; normal range: 115-359 U/L), γ-glutamyl transpeptidase (835 U/L; normal range: 10-47 U/L), amylase (474 U/L; normal range: 37-125 U/L), and C-reactive protein (11.88 mg/dL; normal range: ≤0.3 mg/dL). The levels of tumor markers, such as carcinoembryonic antigen (12.2 ng/mL; normal range: ≤5.0 ng/mL) and carbohydrate antigen 19-9 (3,784 U/mL; normal range: ≤40 U/mL), were also elevated. Abdominal computed tomography (CT) showed the marked distention of the afferent loop and wall thickening at the proximal afferent loop (Fig. 1a) with dilatation of the intrahepatic (Fig. 1b) and extrahepatic bile ducts (Fig. 1c). Multiple liver metastasis and peritoneal dissemination were observed on CT. We diagnosed the patient with ALO caused by the peritoneal dissemination of gastric cancer. We considered that the obstructive jaundice was caused by ALO because bile drainage was prevented by high pressure in the intra-afferent loop.
Figure 1.
Computed tomography on admission revealed marked distention of the afferent loop and wall thickening at the proximal afferent loop (a: white arrow) with dilatation of the intrahepatic bile duct (b) and extrahepatic bile duct (c: white arrow).
Upper gastrointestinal endoscopy (GIF-2T200; Olympus, Tokyo, Japan) was performed to confirm the obstruction of the afferent loop. The endoscope was inserted into the afferent loop; however, the endoscope was too short to reach the site of the stricture. Endoscopic jejunography revealed an irregular stricture of the afferent loop and dilatation of the distal afferent loop (Fig. 2). Ultrasound-guided percutaneous transhepatic biliary drainage (PTBD) was performed under local anesthesia on the day after the endoscopy. A 7.2 F pigtail catheter was inserted into the afferent loop through the left lobe approach. The dilatation of the distal afferent loop was improved by inserting the PTBD catheter, and the levels of hepatobiliary enzymes decreased. Cholangioduodenography via the PTBD catheter revealed an irregular stricture of approximately 46 mm in length in the afferent loop (Fig. 3). A guidewire was passed through the stricture into the proximal aspect of the obstruction on the 8th day after PTBD. The PTBD route was then dilated to a diameter of 10 F, which was the diameter of the SEMS delivery system. After confirming the stricture in the afferent loop by jejunography, a 22×90 mm SEMS (WallFlex™ Duodenal Stent; Boston Scientific, Natick, USA) was inserted over the guidewire through the PTBD route without difficulty. The SEMS was placed appropriately across the stricture in the afferent loop (Fig. 4).
Figure 2.
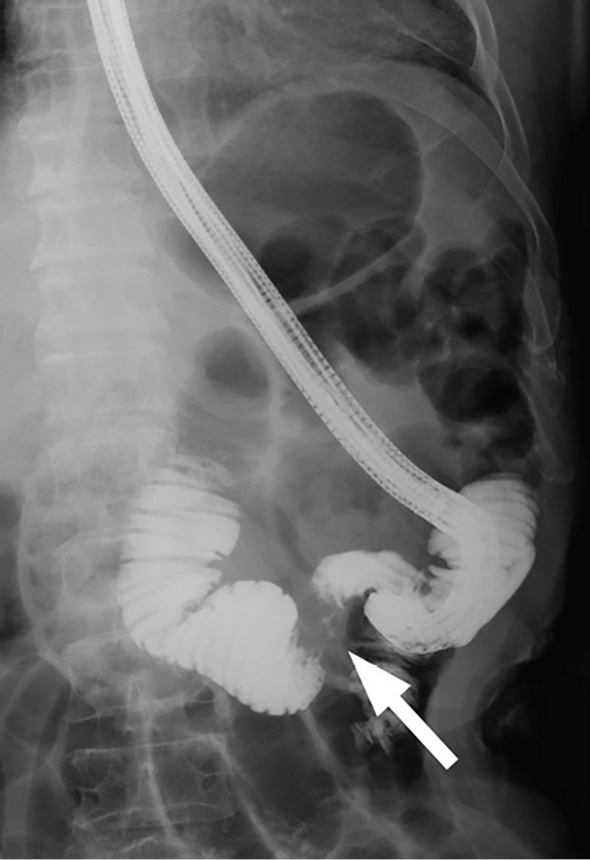
Endoscopic jejunography revealed an irregular stricture of the afferent loop (white arrow) and dilatation of the afferent loop.
Figure 3.
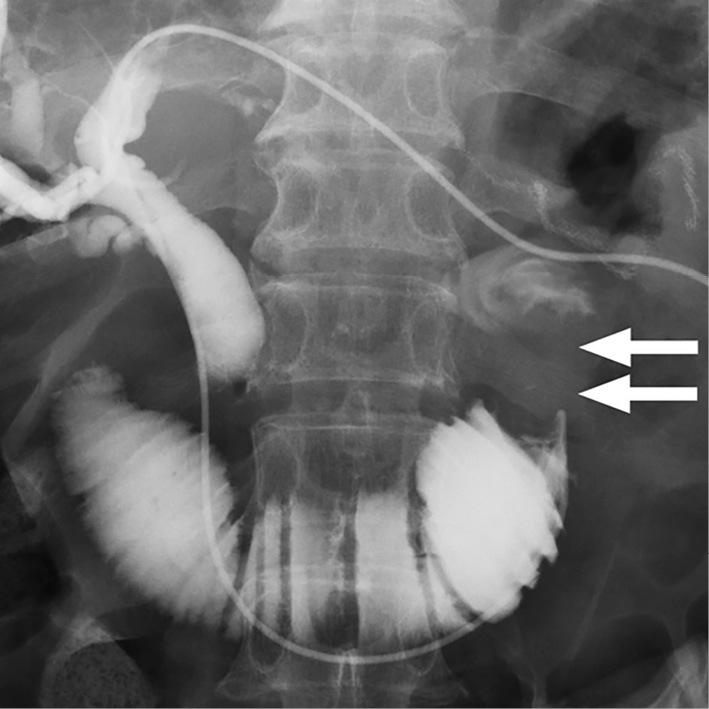
Cholangioduodenography via the PTBD catheter revealed an irregular stricture in the afferent loop that was approximately 46 mm in length (white arrows).
Figure 4.
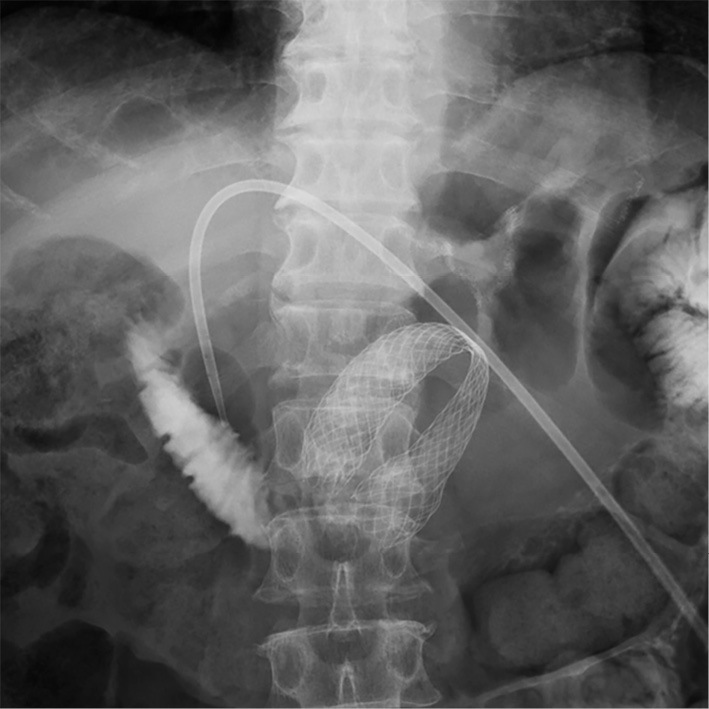
The self-expanding metallic stent was placed across the stricture in the afferent loop.
The patient recovered immediately after the placement of the SEMS without any procedure-related adverse events. The patient started eating 5 days later because he had no symptoms and his laboratory findings were normal. The PTBD tube was removed 14 days after the placement of the SEMS, and the patient was discharged from the hospital. He retained a good quality of life for 3 months after the placement of the SEMS until his death due to the progression of cancer. No obstruction of the SEMS or obstructive jaundice was observed after the placement of the SEMS.
Discussion
ALO is a rare mechanical complication that occurs after gastrojejunostomy, such as Roux-en-Y, Billroth II, Whipple, or Child reconstruction. ALO can be caused by either benign or malignant obstructions, with benign obstruction being the main etiology of ALO. Benign ALO is caused by compression or kinking due to postoperative adhesion, internal herniation, stenosis due to ulceration, or radiation enteritis. In contrast, malignant ALO is caused by the recurrence of cancer in the lymph nodes, peritoneum, gastric remnants, or anastomotic sites. The management strategy depends on the nature of the obstructing lesion (benign or malignant), the obstruction site, and the patency of the primary hepaticojejunostomy and pancreaticojejunostomy. Surgery has an important role in the treatment of benign ALO. However, less-invasive treatments are preferred in patients with malignant ALO due to their poor condition and prognosis (1).
In patients with an advanced malignancy, stent placement is the most appropriate less-invasive therapy for malignant gastrointestinal obstructions from the perspective of quality of life. There are a number of approaches for enteral stent placement in patients with malignant ALO, these include endoscopic, percutaneous transhepatic, and direct percutaneous approaches (1). The endoscopic placement of an SEMS has become a feasible alternative to surgery as a palliative treatment for an inoperable malignant gastric outlet obstruction (13-15). Several cases involving the endoscopic placement of an SEMS for malignant ALO have been reported since the development of DBE- and TTS-type SEMSs (2-5). A few reports have described a combined method in which a DBE is used with an overtube because the large diameter of the SEMS delivery system does not allow for stent deployment through the 2.8-mm working channel of a conventional short DBE (2, 3). However, the combined use of the new short-type DBE with a 3.2 mm working channel and TTS SEMS enables easy SEMS placement (5). Thus, endoscopic SEMS placement is the preferred therapy for malignant ALO.
DBE is useful for treating malignant ALO. However, it is not available in all institutions, and the site of the ALO stricture cannot be reached in all cases even when using a DBE. Alternative methods must be selected to manage malignant ALO when the endoscopic approach is unsuccessful. Percutaneous transhepatic and direct percutaneous approaches are alternative options in these cases (6). In some cases, ALO causes obstructive jaundice and cholangitis. PTBD is an established method for biliary drainage in patients with dilatation of the intrahepatic bile duct. In contrast, the direct puncture of a percutaneous afferent loop is associated with the risk of bile leakage and subsequent peritonitis. Thus, the percutaneous transhepatic approach is the first choice of alternative therapy in these cases. In the present case, percutaneous transhepatic stent placement was attempted rather than permanent PTBD because the patient’s quality of life would be better preserved by placing an internal stent. Actually, we placed the SEMS in the afferent loop through the percutaneous transhepatic route without any complications. To our knowledge, there are eight reported cases in which transhepatic SEMS placement was used for the management of malignant ALO. We have summarized the eight cases and the current case in Table (6-12).
Table.
Reported Cases of Percutaneous Transhepatic SEMS Placement for Malignant ALO.
| Case | Reference | Year | Age (y) | Gender | Etiology | Reconstruction | SEMS | Delivery |
|---|---|---|---|---|---|---|---|---|
| 1 | 7 | 2000 | 65 | M | Gastric cancer | NA | Wallstent 22×100 mm, (uncovered) | 10Fr |
| 2 | 8 | 2000 | 47 | M | Gastric cancer | Roux-en-Y | Wallstent 10×70 mm (uncovered) | NA |
| 3 | 9 | 2003 | 62 | M | Pancreatic cancer | Whipple | Wallstent NA×60 mm (uncovered) | 10Fr |
| 4 | 10 | 2005 | 77 | M | Gastric cancer | Billroth II | SMART stent 10×80 mm SMART stent 10×40 mm (uncovered) |
7Fr×2 |
| 5 | 11 | 2007 | 46 | F | Gastric cancer | Billroth II | Nitinol SEMS 18×80 mm (partially covered) | 12Fr |
| 6 | 11 | 2007 | 60 | M | Gastric cancer | Billroth II | Nitinol SEMS 18×80 mm, (partially covered) | 12Fr |
| 7 | 6 | 2010 | 71 | M | Ampullary carcinoma | Whipple | ComVi 22×100 mm (covered) | 10.5Fr |
| 8 | 12 | 2012 | 51 | M | Ampullary carcinoma | Child | WallFlex 22×120 mm (uncovered) | 10Fr |
| 9 | Current case | 2017 | 71 | M | Gastric cancer | Roux-en-Y | WallFlex 22×90 mm (uncovered) | 10Fr |
An enteral SEMS was used for malignant ALO in six of the previous eight cases, and a 10-mm biliary SEMS were used in two cases. A small-diameter SEMS can easily cause stent dysfunction due to tumor ingrowth or stent migration; thus, in most recent cases involving enteral TTS-type SEMS placement, the diameter of the delivery system was 10 F. There is a large difference in the diameter of the delivery systems that are used to place biliary SEMSs and enteral TTS-type SEMSs. Thus, enteral SEMSs should be preferred because they last longer. In cases without a choledochojejunostomy, pancreatitis is a possible adverse event, because the SEMS is inserted through the duodenal papilla. The development of pancreatitis after the placement of an SEMS through the duodenal papilla was not reported in any of the five previous cases or the present case; however, the possibility of pancreatitis should be considered when using the percutaneous transhepatic approach.
Endoscopic ultrasound (EUS) drainage was developed as an additional option to percutaneous drainage and endoscopic retrograde cholangiopancreatography (ERCP). EUS-guided biliary interventions have been considered to be effective alternatives after a failed transpapillary approach in patients with an altered anatomy. There has only been one report of malignant ALO in which EUS-hepaticogastrostomy was effective after the failure of gastrointestinal stent placement (16). Furthermore, enteral SEMS placement using an EUS-guided antegrade technique might be a promising alternative treatment for patients with malignant ALO.
In summary, we described a case of malignant ALO in which percutaneous transhepatic SEMS placement was effective. The endoscopic placement of an SEMS using a DBE is preferred for malignant ALO. However, percutaneous transhepatic SEMS placement might be an alternative option for the treatment of patients with obstructive jaundice or acute cholangitis in whom an endoscopic approach proves difficult.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Blouhos K, Boulas KA, Tsalis K, Hatzigeorgiadis A. Management of afferent loop obstruction: Reoperation or endoscopic and percutaneous interventions? World J Gastrointest Surg 7: 190-195, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sasaki T, Isayama H, Kogure H, et al. . Double-balloon enteroscope-assisted enteral stent placement for malignant afferent-loop obstruction after Roux-en-Y reconstruction. Endoscopy 46 (Suppl 1 UCTN): E541-E542, 2014. [DOI] [PubMed] [Google Scholar]
- 3. Fujii M, Ishiyama S, Saito H, et al. . Metallic stent insertion with double-balloon endoscopy for malignant afferent loop obstruction. World J Gastrointest Endosc 7: 665-669, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sakai A, Shiomi H, Okabe Y, et al. . Effectiveness of endoscopic self-expandable metal stent placement for afferent loop obstruction caused by pancreatic cancer recurrence after pancreaticoduodenectomy. Clin J Gastroenterol 8: 103-107, 2015. [DOI] [PubMed] [Google Scholar]
- 5. Shimatani M, Takaoka M, Tokuhara M, et al. . Through-the-scope self-expanding metal stent placement using newly developed short double-balloon endoscope for the effective management of malignant afferent-loop obstruction. Endoscopy 48 (Suppl 1 UCTN): E6-E7, 2016. [DOI] [PubMed] [Google Scholar]
- 6. Laasch HU. Obstructive jaundice after bilioenteric anastomosis: transhepatic and direct percutaneous enteral stent insertion for afferent loop occlusion. Gut Liver 4 (Suppl 1): S89-S95, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Caldicott DG, Ziprin P, Morgan R. Transhepatic insertion of a metallic stent for the relief of malignant afferent loop obstruction. Cardiovasc Intervent Radiol 23: 138-140, 2000. [DOI] [PubMed] [Google Scholar]
- 8. Ozden I, Poyanli A, Kaygusuz A, Rozanes I, Alper A. The transhepatic route for the placement of a duodenojejunal stent: application in a postoperative closed loop obstruction of the duodenum. Cardiovasc Intervent Radiol 24: 70-71, 2001. [DOI] [PubMed] [Google Scholar]
- 9. Johnsson E, Delle M, Lundell L, Liedman B. Transhepatic placement of an enteral stent to treat jaundice in a tumor recurrence obstructed afferent loop after a whipple procedure. Dig Surg 20: 329-331, 2003. [DOI] [PubMed] [Google Scholar]
- 10. Yoshida H, Mamada Y, Taniai N, et al. . Percutaneous transhepatic insertion of metal stents with a double-pigtail catheter in afferent loop obstruction following distal gastrectomy. Hepatogastroenterology 52: 680-682, 2005. [PubMed] [Google Scholar]
- 11. Gwon DI. Percutaneous transhepatic placement of covered, self-expandable nitinol stent for the relief of afferent loop syndrome: report of two cases. J Vasc Interv Radiol 18: 157-163, 2007. [DOI] [PubMed] [Google Scholar]
- 12. Hosokawa I, Kato A, Shimizu H, Furukawa K, Miyazaki M. Percutaneous transhepatic metallic stent insertion for malignant afferent loop obstruction following pancreaticoduodenectomy: a case report. J Med Case Rep 6: 198, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Baron TH. Expandable metal stents for the treatment of cancerous obstruction of the gastrointestinal tract. N Engl J Med 344: 1681-1687, 2001. [DOI] [PubMed] [Google Scholar]
- 14. Yim HB, Jacobson BC, Saltzman JR, et al. . Clinical outcome of the use of enteral stents for palliation of patients with malignant upper GI obstruction. Gastrointest Endosc 53: 329-332, 2001. [DOI] [PubMed] [Google Scholar]
- 15. Dormann A, Meisner S, Verin N, Wenk Lang A. Self-expanding metal stents for gastroduodenal malignancies: systematic review of their clinical effectiveness. Endoscopy 36: 543-550, 2004. [DOI] [PubMed] [Google Scholar]
- 16. Ratone JP, Caillol F, Bories E, Pesenti C, Godat S, Giovannini M. Hepatogastrostomy by EUS for malignant afferent loop obstruction after duodenopancreatectomy. Endosc Ultrasound 4: 250-252, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]