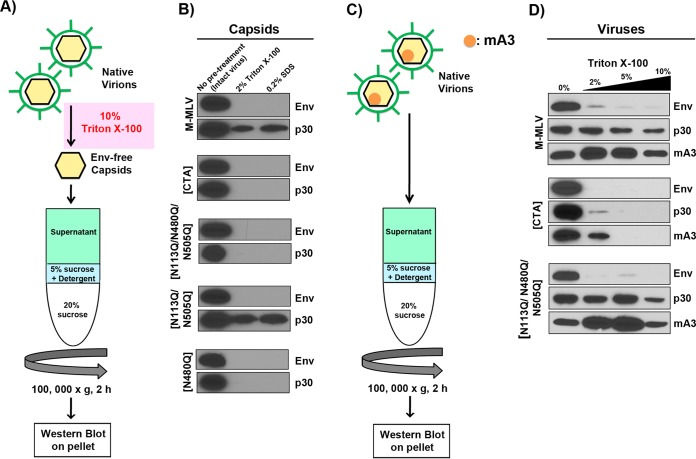
FIG 2.
Glycosylation mutants have diminished capsid stability. (A) Schematic representation of the procedure used for assessment of the stability of envelope-free viral cores in the presence of mild detergent. Native enveloped viruses were first treated with 10% Triton X-100 to strip the viral envelope. Envelope-free viral cores were then submitted to velocity sedimentation through a 5% sucrose step containing either 2% Triton X-100 or 0.2% SDS and then through a 20% sucrose step. (B) Western blots were carried out on intact core pellets by using monoclonal anti-p30 (R187) and anti-EGFP antibodies. EGFP was expressed as a fusion protein with the viral envelope glycoprotein (Env). (C) Schematic representation of the methodology used to assess packaging of mA3 inside viral cores. Native, enveloped virions were submitted to velocity sedimentation through a 5% sucrose step containing 0%, 2%, 5%, or 10% Triton X-100 and then through a 20% sucrose step. (D) Western blots were carried out on intact viral cores as described above; mA3 was detected using an anti-FLAG monoclonal antibody.