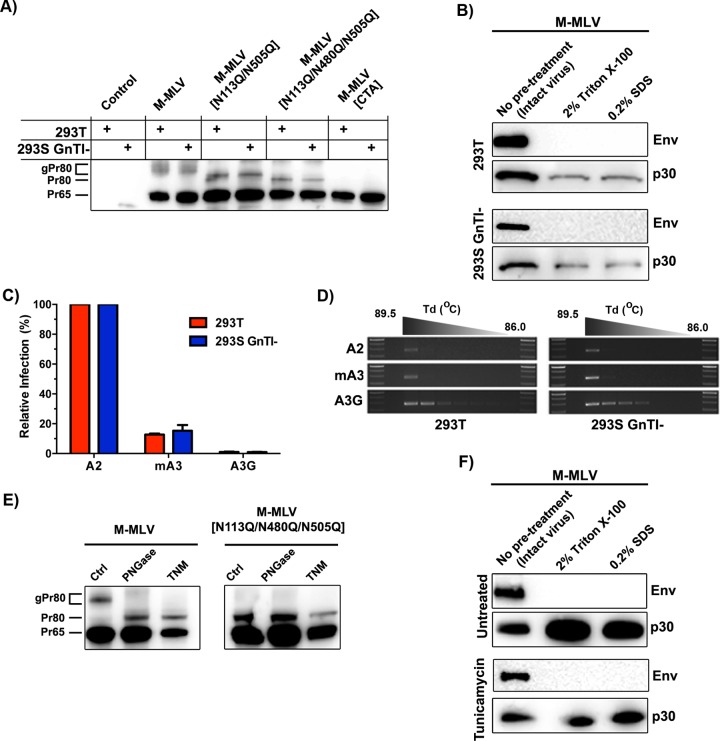
FIG 4.
Influence of gPr80 glycosylation on capsid stability and resistance to mA3 deamination. (A) Lysates from control (untransfected) or transfected 293T and 293S GnTI− cells were analyzed by SDS-PAGE. Protein expression was detected using the polyclonal anti-p30 antibody. (B) Viruses were processed as described in the legend to Fig. 2B. Western blots were carried out using monoclonal anti-p30 and anti-EGFP antibodies. EGFP was expressed as a fusion protein with the viral envelope glycoprotein (Env). (C) 293T and 293S GnTI− cells were cotransfected with M-MLV and APOBEC expression plasmids, and supernatants were harvested 48 h later. Infection in NIH 3T3 cells was measured by EGFP expression 48 h later. (D) Deamination intensity analysis by 3D-PCR. The analysis was performed on genomic DNAs extracted from the infected NIH 3T3 cells used for panel C. The results show a 279-bp product of the EGFP gene that was amplified using a decreasing denaturing temperature gradient (Td) from 89.5°C to 86.0°C. Larger numbers of bands indicate more intensely mutated products. (E) Western blot analysis of gPr80 expression in tunicamycin-treated cells. Untreated cells (left lanes) and PNGase F-treated lysates (middle lanes) were compared to tunicamycin-treated (TNM) cells (right lanes) for both M-MLV and M-MLV[N113Q/N480Q/N505Q]. (F) Capsid stability of viruses produced in tunicamycin-treated cells, determined as described for panel B.