FIG 1.
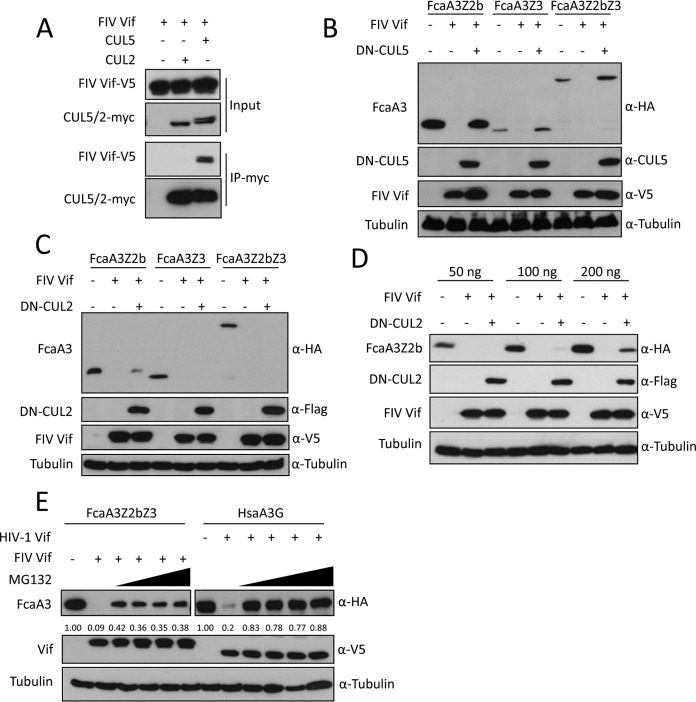
CUL5 is required for FIV Vif-induced degradation of feline APOBEC3s. (A) FIV Vif interacts with CUL5 but not CUL2. myc-CUL5 or myc-CUL2 expression plasmids were cotransfected with the FIV Vif-V5 expression plasmid. Cell lysates were immunoprecipitated with anti-myc beads and then analyzed by immunoblotting with anti-V5 antibody for FIV Vif and anti-myc antibody for CUL2 and CUL5. (B and C) Dominant negative CUL5 (DN-CUL5), but not DN-CUL2, disrupts the degradation of feline A3s induced by FIV Vif. HEK293T cells were cotransfected with 300 ng expression plasmids for FcaA3Z2b-HA, FcaA3Z3-HA, or FcaA3Z2bZ3-HA and 700 ng of DN-CUL5–FLAG or DN-CUL2–FLAG with 30 ng of FIV Vif-V5. pcDNA3.1 was used as a control plasmid to replace the FIV Vif or dominant negative CUL5/2 expression plasmids. Cells were analyzed by immunoblotting using anti-HA, anti-V5, anti-CUL5, anti-Flag, and antitubulin antibodies. (D) HEK293T cells were cotransfected with 50, 100, or 200 ng expression plasmids for FcaA3Z2b-HA and 700 ng of DN–CUL2-FLAG with 30 ng of FIV Vif-V5. Immunoblotting was performed as described above for panel B. (E) FIV Vif induces the degradation of FcaA3s in a proteasome-dependent manner. HEK293T cells were transfected with HsaA3G-HA or FcaZ2bZ3-HA and HIV-1 Vif-V5 or FIV Vif-V5 expression plasmids; pcDNA3.1 was used as an empty plasmid control. The transfected cells were treated with the proteasome inhibitor MG132 (2.5, 5, 7.5, or 10 μM) or DMSO as a control at 36 h posttransfection. Cells were harvested 12 h later (48 h after transfection) and then analyzed by immunoblotting with anti-HA, anti-V5, and antitubulin antibodies. The percentages of FcaA3 and HsaA3G were calculated relative to those in the absence of FIV Vif or HIV-1 Vif (set as 1.00).