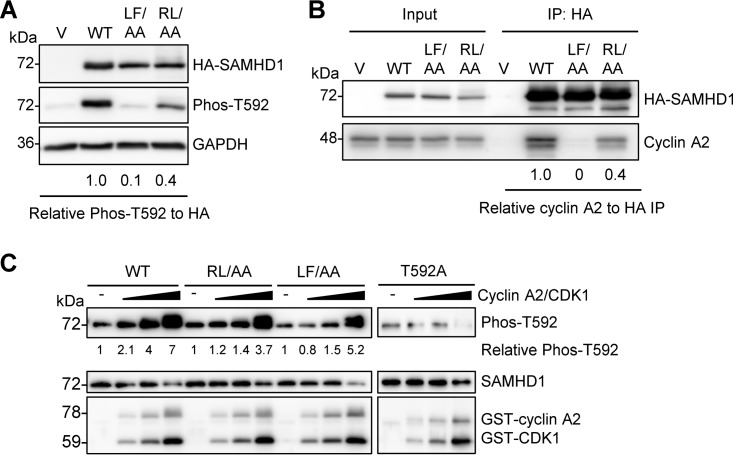
FIG 3.
RXL mutation of SAMHD1 reduces T592 phosphorylation and cyclin A2 binding. (A) HEK293T cells expressing vector or WT, LF/AA, or RL/AA SAMHD1 protein were analyzed by immunoblotting for Phos-T592, HA-SAMHD1, or GAPDH. Relative Phos-T592 was calculated by normalizing densitometry values for Phos-T592 and total SAMHD1 (HA) signals to GAPDH. The relative ratio of Phos-T592 to total SAMHD1 is shown; the value for the WT was set as 1. Immunoblot data are representative of two independent experiments; for densitometry, the average of two experiments is depicted. (B) Co-IP of cyclin A2 from HEK293T cells overexpressing vector or WT, LF/AA, or RL/AA SAMHD1 protein. Transfected cells were lysed and HA-tagged SAMHD1 was bound to HA-conjugated agarose. SAMHD1-interacting proteins were eluted and analyzed by immunoblotting for HA or cyclin A. Data are representative of results from two independent experiments. Cyclin A2 IP densitometry was normalized to HA-SAMHD1 IP products. Relative amounts were calculated according to the value for WT SAMHD1, which was set as 1. (C) Recombinant WT, RL/AA, LF/AA, or T592A SAMHD1 protein was used for in vitro kinase assays. SAMHD1 proteins were incubated with increasing amounts of cyclin A2/CDK1 complex in the presence of ATP. Kinase-free samples were used as a negative control. Reactions were analyzed by SDS-PAGE and immunoblotting for SAMHD1, Phos-T592, and GST. Data are representative of results from three independent experiments. The densitometry value of the T592 phosphorylated species of SAMHD1 was calculated, with the value for the kinase-free samples set as 1 and each protein phosphorylation calculated relative to the kinase-free control.