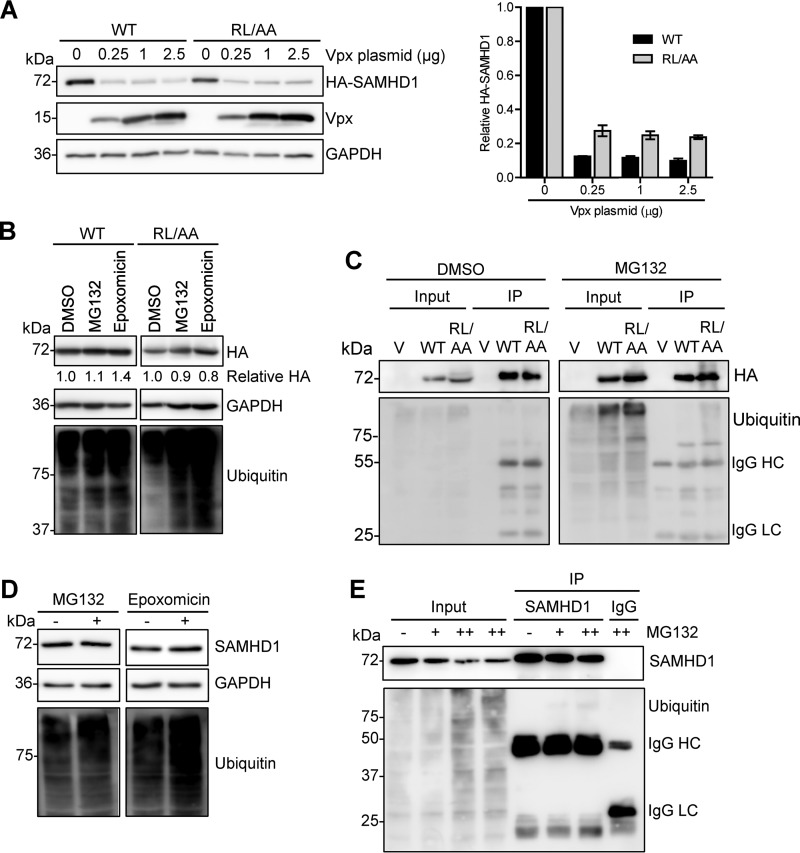
FIG 5.
WT SAMHD1 and RL/AA mutant proteins are degraded by Vpx from HIV-2 but are not spontaneously ubiquitinated. (A) HEK293T cells were cotransfected with plasmids expressing WT (0.1 μg) or RL/AA (1 μg) SAMHD1 along with a plasmid expressing HIV-2ROD Vpx. Empty vectors (pLenti-puro and pCG-myc) were used to normalize all DNA transfection amounts. Lysates were harvested and HA-tagged SAMHD1 and Vpx expression was confirmed by immunoblotting using anti-HA and Vpx-specific antibodies, respectively. GAPDH was used as a loading control. Relative SAMHD1 protein levels were calculated by densitometry and normalized to the value for GAPDH. The value for cells not treated with Vpx was set as 1. The graph depicting densitometry shows averaged data from two independent experiments. (B) HEK293T cells transfected with plasmid DNA expressing WT or RL/AA SAMHD1 were pretreated with MG132 (1 μM) or epoxomicin (1 μM) for 3 h. Cells were washed and fresh medium was added without inhibitors. Lysates were harvested 6 h after the removal of MG132. Lysates were immunoblotted for expression of HA and ubiquitin, and GAPDH was used as a loading control. Relative SAMHD1 was calculated as described for panel A. The value for DMSO controls was set as 1. (C) HEK293T cells expressing WT or RL/AA SAMHD1 were treated with MG132 (1 μM) and cells were lysed in lysis buffer containing protease inhibitor MG132 and N-ethylmaleimide. HA-IP was performed and IP products were analyzed by immunoblotting. Cells transfected with plasmid expressing empty vector were used as a negative control. IgG heavy chain (HC) and light chain (LC) contaminating bands are indicated in panels C and E. (D) THP-1 cells were pretreated with MG132 (0.5 μM) or epoxomicin (0.1 μM) for 3 h. Cells were washed and fresh medium without inhibitors was added. Cell lysates were harvested 6 h after the removal of MG132. (E) THP-1 cells were treated with MG132 for 12 h (0.1 or 1 μM, indicated by “+” and “++,” respectively). Cells were lysed in cell lysis buffer as described for panel C. SAMHD1 IP was performed with the cell lysates. Nonspecific mouse IgG was used as a negative control. IP products were analyzed by immunoblotting with antibodies specific to SAMHD1 and ubiquitin.