Figure 1.
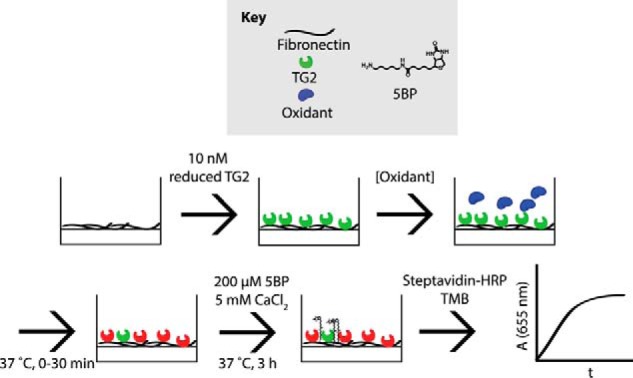
Oxidation of immobilized TG2 activity assay. Reduced, recombinant TG2 (green) was immobilized onto fibronectin-coated plates and incubated with oxidants (blue) for up to 30 min at 37 °C, producing inactivated TG2 (red). Oxidants were then washed away, and 200 μm 5BP and 5 mm CaCl2 were added to analyze TG2 activity. 5BP is a biotinylated primary amine that is a good substrate of TG2 and is therefore cross-linked to Gln residues on extracellular matrix proteins, such as fibronectin, in the presence of catalytically active extracellular TG2 (26). Streptavidin-HRP binds to cross-linked 5BP and turns over TMB, producing a blue color that can be spectrophotometrically monitored at 655 nm. The steady-state slope was extracted and was proportional to TG2 activity.
