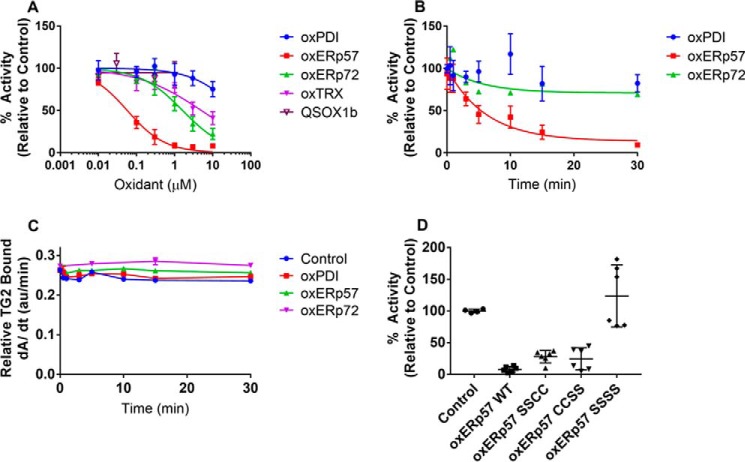
Figure 6.
ERp57 specifically oxidizes TG2 in vitro. A, dose dependence of TG2 oxidation by CXXC proteins. 10 nm reduced TG2 was immobilized onto fibronectin and incubated with each oxidized CXXC protein for 30 min at 37 °C. After a series of washes with PBS, 200 μm 5BP and 5 mm CaCl2 were added to probe for TG2 activity (see Fig. 1). Experiments were performed in quadruplicate with three technical replicates per concentration. B, time dependence of TG2 oxidation by CXXC proteins. 10 nm reduced TG2 was immobilized onto fibronectin and oxidized with 1 μm PDI, ERp57, or ERp72 for up to 30 min at 37 °C. After a series of PBS washes, 200 μm 5BP and 5 mm CaCl2 was added to probe for TG2 activity. Experiments were performed in triplicate with three technical replicates per time point, and the first-order kinetic profile was fit to obtain an apparent rate constant, kapp. The bimolecular rate constant was derived by dividing the kapp by the initial oxidant concentration (1 μm). C, TG2 remains bound to fibronectin even after oxidation by PDI, ERp57, or ERp72. Immobilized TG2 was incubated with each oxidant for up to 30 min at 37 °C and probed with a rabbit anti-TG2 antibody. The amount of bound TG2 was determined by incorporation of an anti-rabbit HRP-conjugated secondary antibody and TMB liquid substrate system. TMB turnover was monitored continuously for 30 min, and the steady-state slope from 0 to 5 min is presented. D, either CXXC active site of ERp57 can oxidatively inactivate TG2. ERp57 mutants were generated to inactivate the redox-active Cys residues in domain a (SSCC), domain a′ (CCSS), or both domains (SSSS). 10 nm reduced TG2 was immobilized onto fibronectin, and incubated with oxidized proteins for 30 min at 37 °C. After a series of PBS washes, 200 μm 5BP, and 5 mm CaCl2 was added to probe for TG2 activity. Experiments were performed in duplicate with three technical replicates per condition. Data are presented as average ± S.D.