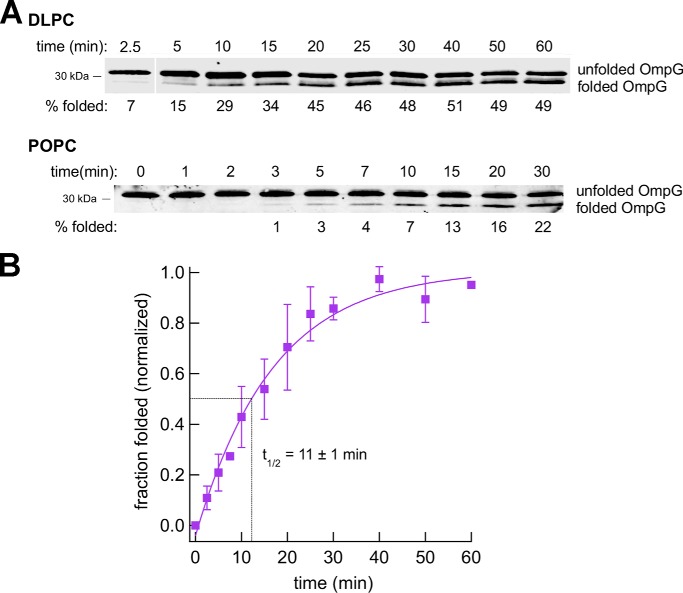
Figure 6.
Bam complex–mediated assembly of OmpG is relatively slow. Urea-denatured OmpG was incubated with SurA and proteoliposomes that contained the Bam complex and either DLPC or POPC at 30 °C, and the folding of the protein after 0–60 min was monitored by Western blotting using an anti-OmpG antiserum. Representative results are shown in A. Molecular weight markers were run to the right of the 2.5-min time point on the top gel and have been deleted for clarity. The assembly of OmpG into proteoliposomes that contained DLPC was quantitated in B. The data were normalized to the maximum fraction of the protein that folded during each assembly reaction, which was defined as 1.0. Average values from multiple experiments (squares) and S.D. values (error bars) at each time point are shown. The time required to reach 50% maximal assembly (t½) was calculated from a single-exponential fit.