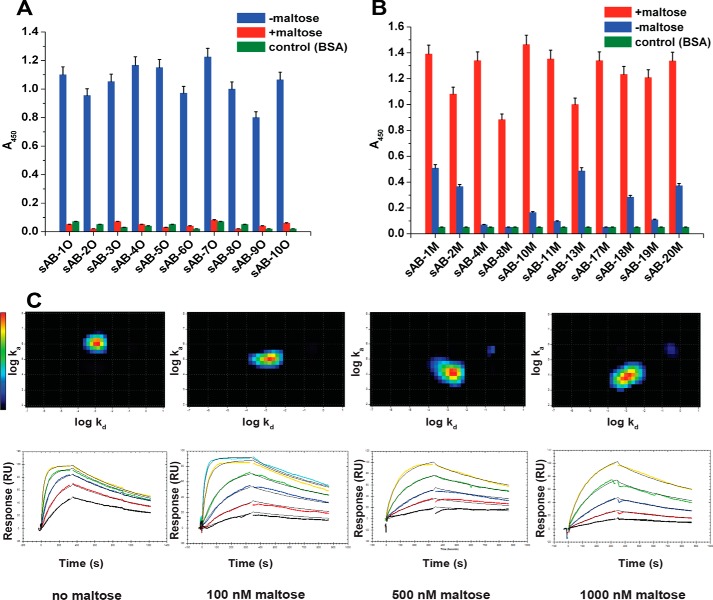
Figure 1.
Characterization of conformation-specific sABs. Phage ELISA shows that sABs generated against the open form of MBP in the absence of maltose do not bind to the closed maltose-bound form of MBP (A) and sABs generated against the maltose-bound, closed form of MBP do not bind or bind with very reduced affinity to the open form of MBP (B). C, kinetics of sAB-7O binding to MBP at different maltose concentration. The kinetics is on-rate–dependent, and affinity decreases steadily with increasing maltose concentration. Interaction Map shows a homogeneous 1:1 interaction. Change in relative position of the heat map on the y axis (log ka) is clear due to a decrease in the on-rate with maltose concentration, whereas the position on the x axis (log kd) remains constant due to invariance in the off-rate. Error bars, S.E.