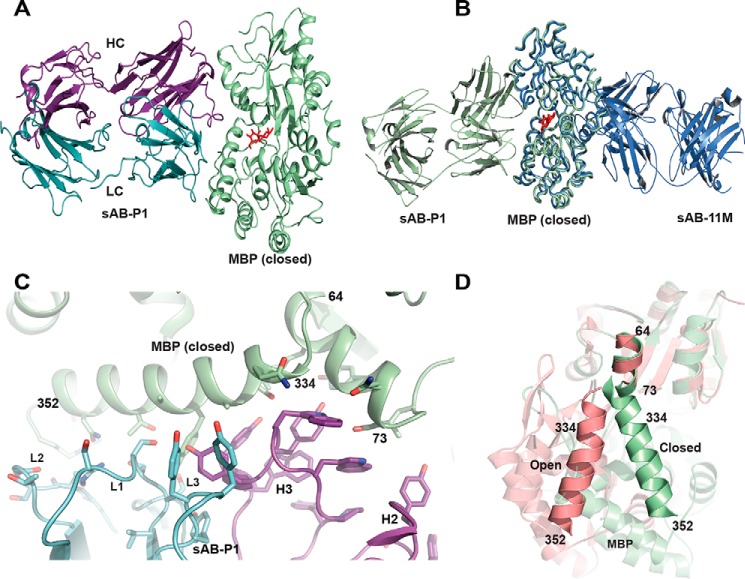
Figure 6.
Structural features of sAB-P1·MBP complex. A, sAB-P1 binds to the closed conformation of MBP interacting across the face of the binding pocket with the maltose trapped inside. B, superposition of MBP bound to sAB-P1 (green) with that of sAB-11M (slate) shows that sAB-P1 binds to the peristeric site of MBP in the closed form opposite to sAB-11M. C, interactions of the HC (magenta) and LC (blue) CDRs of sAB-P1 with the two helices (64–73 and 334–352) of MBP (green) at the peristeric interface. D, the relative orientations of the helices comprising residues 64–73 and 341–350 are different in open (salmon) and closed (green) forms of MBP. These helices in the closed conformation interact extensively with sAB-P1 (C), thereby imparting conformational specificity.