Figure 7.
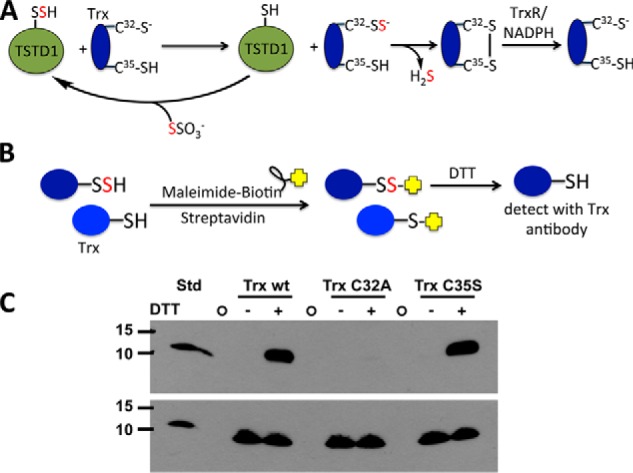
Mechanism of TSTD1-dependent persulfidation of thioredoxin and its detection by the biotin thiol assay. A, mechanism of sulfur transfer from thiosulfate to thioredoxin (Trx) via TSTD1. Cys-32 is the nucleophilic cysteine, and Cys-35 is the resolving cysteine. Oxidized thioredoxin is recycled by thioredoxin reductase (TrxR) and NADPH. B, strategy for detection of persulfidated thioredoxin using the biotin thiol assay. Reactive and accessible persulfide and thiol groups on thioredoxin are alkylated by maleimide biotin and immobilized on a streptavidin column. Following DTT treatment, only thioredoxin, which had a persulfide group, is released from the column and can be detected by immunoblotting. C, Western blot analysis of TSTD1-dependent persulfidation of thioredoxin from the biotin thiol assay. A representative Western blot is shown in which elution of wildtype thioredoxin (Trx wt) and the C35S but not the C32A mutant was detected following DTT treatment of the loaded streptavidin column. The bottom panel represents an equal loading control for the presence of thioredoxin in each sample before loading on the streptavidin column. The first lane contains purified wildtype thioredoxin (Trx wt STD; 35 ng). The circles represent empty lanes. The position of the molecular mass markers (in kDa) is shown on the left.
