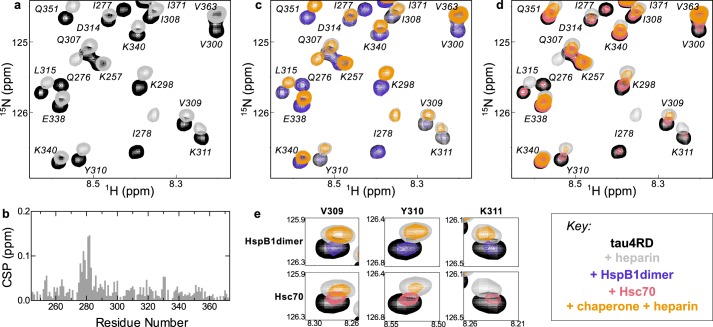
Figure 6.
Effects of heparin on chaperone binding. a, 15N HSQC TROSY of 15N-labeled tau4RD alone (black) and in the presence of 2.4 molar eq of heparin (gray). b, chemical-shift perturbations (CSP) of each peak in A plotted as a function of residue number. The highest chemical-shift perturbations map to the aggregation-prone motif at the start of the second repeat of tau4RD, 275VQIINK280. c, 15N HSQC TROSY of 15N-labeled tau4RD alone (black), in the presence of 2.4 molar eq of heparin (gray), in the presence of 2 molar eq of HspB1dimer (blue), and in the presence of both 2.4 eq of heparin and 2 eq of HspB1dimer (yellow). HspB1dimer binding is largely unchanged by the presence of heparin. d, 15N HSQC TROSY of 15N-labeled tau4RD alone (black), in the presence of 2.4 molar eq of heparin (gray), in the presence of 2 molar eq of Hsc70 (red), and in the presence of both 2.4 eq of heparin and 2 eq of Hsc70 (yellow). Hsc70 binding is enhanced by the presence of heparin, as indicated by the enhanced broadening in the yellow spectrum relative to the red. e, comparison of residues Val-309, Tyr-310, and Lys-311 in the HspB1dimer-bound and Hsc70-bound spectra in the presence of heparin. Although HspB1dimer and Hsc70 cause a similar amount of broadening in these peaks in the absence of heparin, in the presence of heparin, Hsc70 causes significantly more broadening in these peaks than HspB1dimer, indicating that heparin enhances Hsc70 binding in a way that it does not for HspB1dimer.