Abstract
Potato starch wastewater was used as fermentation medium for Rhodococcus erythropolis to produce bioflocculant. Kinetics of cell growth and bioflocculant production were firstly constructed. After fermentation for 60 h, 0.97 g of bioflocculant with polysaccharides nature was extracted from 1 L of fermentation liquor. Kinetics characteristics showed that cell growth and bioflocculant production could be simulated well with Logistic and Luedeking-Piret equations, respectively. When R. erythropolis was in logarithm growth phase, COD, ammonium, and TP of the potato starch wastewater medium were rapidly down to 1736, 188, and 146 mg/L, respectively, from 7836, 975, and 712 mg/L, while the medium’s exactly pH value was almost not changed. Furthermore, bioflocculant flocculation can be used as an effective pretreatment way for potato starch wastewater, and it was feasible in actual treatment projects in Ronghua Starch Co., Ltd., Sichuan Province.
Introduction
Flocculation technique, an effective method in aggregating colloids, has been widely used in industrial settings1, for instance, a coagulation–flocculation (CF) process using iron-based coagulants resulted in COD, color, turbidity and humic acids removal of 56.4%, 63.4%, 89.8%, and 70.4% from landfill leachate2; after treated by the composite of bioflocculant and CaCl2, 91.8% of turbidity and 89.7% of COD were removed from domestic wastewater3. Although traditional flocculants were effective and economical in wastewater treatment, their degraded monomers always posed serious health and environmental concerns4,5. Compared with traditional flocculants, bioflocculant, secreted by microorganisms during their active secretion and cell lysis, has been attracted scientific attention due to its biodegradable and less harmful to human and ecosystems. Literatures reported that the bioflocculant was effective in the treatment of low temperature drinking water6, swine wastewater7, and so on, on laboratory scales.
Over time, different kinds of microorganisms and their bioflocculant have been reported, such as Bacillus licheniformis, Lipomyces starkeyi, and Ochrobactium ciceri6,8,9. However, there were no comprehensive studies on variation of the fermentation medium’s composition, which directly related to the characteristics of cell growth, metabolites generation, and fermentation kinetics10. It is precisely that the investigation of these elements would be beneficial for improving bioflocculant yield and flocculating activities in practical application10. Besides, high production cost was still the major limitation for bioflocculant in large scale production and commercial applications, and so far, with the aim of commercialization, a considerable effort has gone into reducing the production cost through using some wastes rich in organic matter, nitrogen, and phosphorus. For instance, Guo et al. produced bioflocculant by using swine wastes and rice stover11, Wang et al. produced bioflocculant by using dairy wastewater12, More et al. produced bioflocculant by using excess sludge13.
Potato starch wastewater, usually produced in the manufacturing process of potato starch and some related products, was one of the most serious and unwieldy pollution sources in food industry, in which the large amount of organic pollutants could be used to cultivate microorganisms to produce bioflocculant14. Utilization of potato starch wastewater to produce bioflocculant, can reduce the production cost, and further reduce the pollution caused by uncontrolled emissions of this type of wastewater.
This study effectively applied R. erythropolis to produce bioflocculant by using potato starch wastewater. Based on the optimization of the fermentation process, kinetics of cell growth and bioflocculant production were simulated. Meanwhile, concentrations of COD, ammonium, total phosphorus (TP), and pH value of the potato starch wastewater medium were examined. Furthermore, actual applications of the bioflocculant were discussed.
Results
Optimization of the fermentation process
Reference to our previous study about the cultivation of Paenibacillus polymyxa15, effects of extra phosphate salts, nitrogen source, carbon source, and initial pH value of the potato starch wastewater medium on bioflocculant production were investigated in turn. From Figs 1–4, it is clearly showed that extra phosphate salts (at different dose) and all of the selected inorganic and organic nitrogen sources (including 2 g/L of (NH4)2SO4, urea, and yeast extract) were beneficial for bioflocculant yield and flocculating activity, while extra carbon sources (including 2 g/L of glucose and sucrose, and 2 mL/L of methanol and 95% ethanol) almost have no promotion, compared with the fermentation liquor from the potato starch wastewater medium (0.12 g/L of bioflocculant and 66.8% of flocculating activity can be obtained). Especially, the bioflocculant yield and flocculating activity improved to 0.75 g/L and 78.4% respectively when the total added phosphate salts were 6 g/L (4 g/L of K2HPO4 and 2 g/L KH2PO4), which were further improved to 0.96 g/L and 92.8% respectively when 2 g/L urea was extra added as the nitrogen source. The role of carbon during the bioflocculant production was inconsistent with conclusions reported by Xia et al. and Li et al., in which glucose was the most preferred carbon source for Proteus mirabilis and Bacillus licheniformis6,16. This may be due to the high-levels of organics in potato starch wastewater. When adjusted the initial pH value in range of 6.5–8.5, R. erythropolis can effectively produce bioflocculant, especially at pH point of 7.5, bioflocculant yield and flocculating activity were appeared as 1.18 g/L and 95.6%, respectively. It is noteworthy that pH range of 6.5–8.5 just covered the pH value of the potato starch wastewater (6.8), so, pH adjustment was not needed from a practical standpoint.
Figure 2.
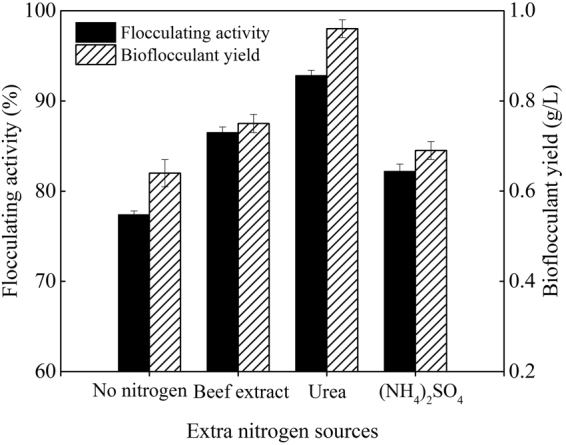
Effects of extra nitrogen sources on bioflocculant yield and its flocculating activity (Major experimental conditions: R. erythropolis was incubated in the potato starch wastewater with 4 g/L K2HPO4, 2 g/L KH2PO4, 0.2 g/L MgSO4, 0.1 g/L NaCl, and 2 g/L of different extra nitrogen, with pH value of 6.8, at 150 r/min and 30 °C).
Figure 3.
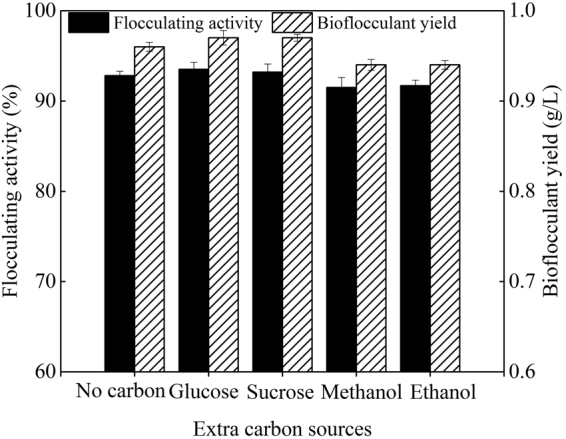
Effects of extra carbon sources on bioflocculant yield and its flocculating activity (Major experimental conditions: R. erythropolis was incubated in the potato starch wastewater with 4 g/L K2HPO4, 2 g/L KH2PO4, 0.2 g/L MgSO4, 0.1 g/L NaCl, 2 g/L urea, and 2 g/L of different extra carbon, with pH value of 6.8, at 150 r/min and 30 °C).
Figure 1.
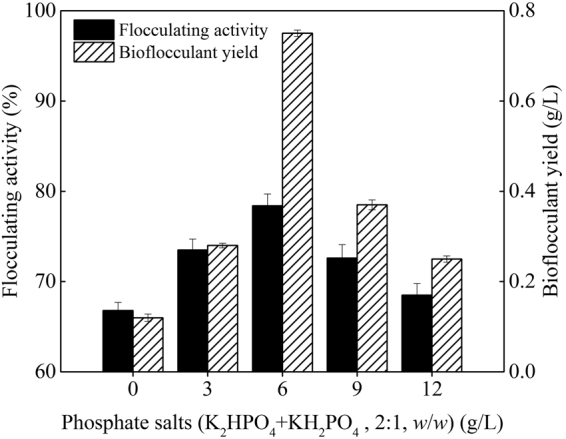
Effects of phosphate salts on bioflocculant yield and its flocculating activity (Major experimental conditions: R. erythropolis was incubated in the potato starch wastewater with 0.2 g/L MgSO4, 0.1 g/L NaCl, and different concentration of phosphate salts, with pH value of 6.8, at 150 r/min and 30 °C).
Figure 4.
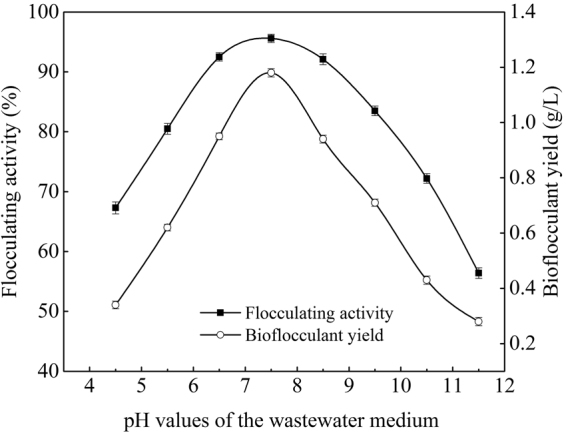
Effects of pH value of the wastewater medium on bioflocculant yield and its flocculating activity (Major experimental conditions: R. erythropolis was incubated in the potato starch wastewater medium of composition: 1 L potato starch wastewater, 4 g K2HPO4, 2 g KH2PO4, 0.2 g MgSO4, 0.1 g NaCl, and 2 g urea, with different pH value (4.5–11.5), at 150 r/min and 30 °C).
Time course of cell growth and bioflocculant yield
As seen from the cell growth curve in Fig. 5, after cultivation for 60 h, cell dry weight, cell density (OD600), and colonies number were increased rapidly to 1.58 g/L, 0.86 and 5.3 × 107 cfu/mL, respectively. Cells entered stationary phase since 60 h, and on 78 h and onward, the cells were in death phase, cell dry weight and density were still increased, while colonies number was reduced. As known to all, cell dry weight contained both the weight of live and death bacteria, so it was increased. The increasing in cell density was due to the increased turbidity after bacteria died in the medium. Colonies number was expressed in the number of viable cells, which was reduced when the cells were entered into death phase, due to the rapid reduction of carbon source, ammonium, and phosphorus, and the cytotoxicity chemicals derived from the accumulation of metabolites in medium17. From Fig. 6, both of bioflocculant yield and its corresponding flocculating activity were increased along with the fermentation time and reached their peaks of 0.97 g/L and 93.1% at 60 h as well.
Figure 5.
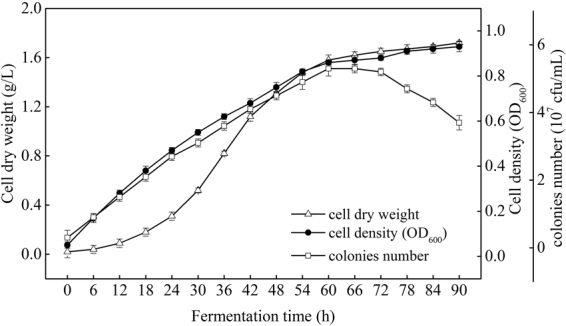
Growth curve of Rhodococcus erythropolis (Major experimental conditions: R. erythropolis was incubated in the potato starch wastewater medium of composition: 1 L potato starch wastewater, 4 g K2HPO4, 2 g KH2PO4, 0.2 g MgSO4, 0.1 g NaCl, and 2 g urea, with pH value of 6.8, at 150 r/min and 30 °C).
Figure 6.
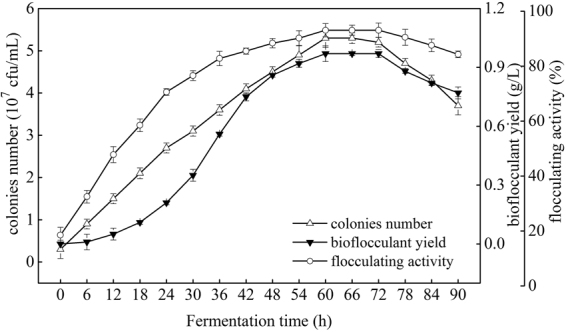
Relations between cell growth and bioflocculant production (Major experimental conditions: R. erythropolis was incubated in the potato starch wastewater medium of composition: 1 L potato starch wastewater, 4 g K2HPO4, 2 g KH2PO4, 0.2 g MgSO4, 0.1 g NaCl, and 2 g urea, with pH value of 6.8, at 150 r/min and 30 °C).
Above results reflected that cell growth as well as bioflocculant yield increased sharply in the period of 0–60 h. Both bioflocculant yield and colonies number were reached their peaks at 60 h and then stabilized after 60 h, indicated that bioflocculant production was almost simultaneously accompanied with cell growth. Afterwards, the bioflocculant yield was decreased monotonically to 0.77 g/L at 90 h, which may be due to cell autolysis and the decrease of enzymatic activity9. A similar bioflocculant production style was observed by Aspergillus parasiticus18.
Analysis of the bioflocculant’s nature
Proportions of total sugar and protein contents were detected as 92.3% and 7.6% (w/w), respectively. Further chemical analysis revealed that the total sugar mainly including 49.7% of neutral sugar, 13.2% of uronic acid, 12.4% of amino sugar, and so on, different from our previous research in which bioflocculant was prepared by using swine wastes11. This was ascribed to the different nutrition components and biophysical environments, which may account for the discrepancy in bioflocculant components19. Gel permeation chromatography indicated that approximate molecular weight of the bioflocculant was 3.85 × 105 Da.
Results of thermal stability test showed that the bioflocculant was thermostable and retained more than 89.7% of flocculating activity in a temperature range of 10–70 °C. Further, residual flocculating activities of 80.3% or more could still be achieved after heating for 30 min at 80, 100, and 120 °C, respectively. These thermo-stable characteristics suggested that active ingredients in the bioflocculant were mainly polysaccharide. Literatures reported that in temperature range of 30–120 °C, polysaccharide chains can remain extended and exposed its flocculating sites, and ensure the flocculating activity, for example, flocculating activity of the bioflocculant produced by Bacillus subtilis remained its 89% at 100 °C20.
Infrared spectrum in Fig. S1 displayed a broad stretching peak at 3430 cm−1, which was assigned to –OH and –NH221, in accordance with that of NOC-1 (mainly glycoprotein)18. The peak around 1632 cm−1 may be assigned to the –CO stretching in –CONH2 group, and the peaks around 1080 cm−1 were characteristic of C–O groups. The peak at 1405 cm−1 is probably an indication of –COO− symmetric stretching vibration. Typical functional groups of polysaccharide, namely, hydroxyl (–OH), amino (–NH2), carboxyl (C–O) groups, and acylamino (–CONH2) groups were all observed in the FTIR spectrum, revealed that the bioflocculant mainly contains polysaccharide. The FTIR spectrum of this bioflocculant was similar to that of Sun et al.’s research, in which functional groups of a polysaccharide flocculant made from sludge cells directly were investigated21. But the bioflocculant was somehow different from the R. erythropolis sludge bioflocculant (RSF), which was prepared by using sludge and livestock wastewater19, even though the FTIR spectrum of the RSF showed the typical functional groups of polysaccharide, the former exhibited absorption at 582 cm−1, corresponding to the existence of protein.
Variation of the medium’s composition
During fermentation process, COD and ammonium contents were rapidly down to 1736 and 188 mg/L from 7836 and 975 mg/L, respectively when R. erythropolis was in logarithm growth phase, while in stationary phase (60–78 h), the down-ward trends of COD and ammonium were became slowly and maintained at a low level at last (Fig. 7). Further, ammonium was recovered slowly after R. erythropolis entered into death phase. From Fig. 8, it is clearly showed that TP concentration was decreased rapidly to 146 mg/L when R. erythropolis was in logarithm growth phase, and the downward trend was just slow after entered into the stationary and death phases. In addition, exactly pH value of the potato starch wastewater medium was increased first and then decreased but the trend was not significant (maintained in the range of 6.4–7.0), due to the buffer effect of phosphorus15.
Figure 7.
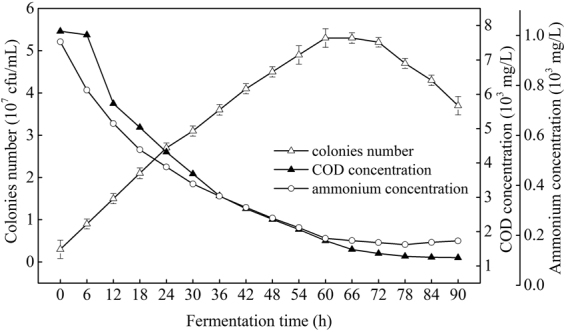
Changes of COD and ammonium with cell growth during the fermentation process (Major experimental conditions: R. erythropolis was incubated in the potato starch wastewater medium of composition: 1 L potato starch wastewater, 4 g K2HPO4, 2 g KH2PO4, 0.2 g MgSO4, 0.1 g NaCl, and 2 g urea, with pH value of 6.8, at 150 r/min and 30 °C).
Figure 8.
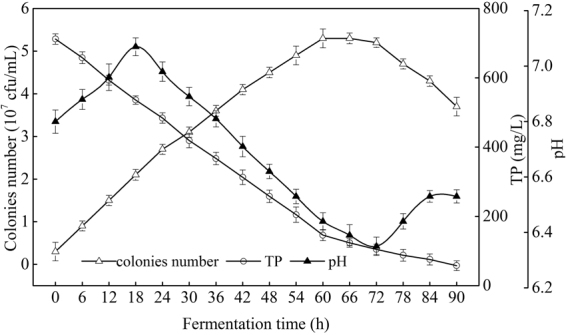
Changes of TP and pH value with cell growth during the fermentation process (Major experimental conditions: R. erythropolis was incubated in the potato starch wastewater medium of composition: 1 L potato starch wastewater, 4 g K2HPO4, 2 g KH2PO4, 0.2 g MgSO4, 0.1 g NaCl, and 2 g urea, with pH value of 6.8, at 150 r/min and 30 °C)
Cell growth kinetics
During fermentation process, the initial and the largest cell dry weights (X0, Xm) were detected as 0.02 and 1.72 g/L, respectively. Cell growth kinetics which described the cell dry weights was obtained from the non-linear fit (Fig. 9) of Eq. (5) and given as follows:
| 1 |
During fermentation process, the initial and the largest biomass concentrations (X0, Xm) were detected as 0.05 and 0.92, respectively. Cell growth kinetics which described the biomass concentrations was obtained from the non-linear fit (Fig. 10) of Eq. (5) and given as follows:
| 2 |
From Table 1, the average relative error between the cell dry weights predicted by the cell growth kinetics model (predicted value) and measured from experiments (actual value) was appeared as 3.98% <10%, similarly, the average relative error between the biomass concentrations predicted by the cell growth kinetics model (predicted value) and measured from experiments (actual value) was appeared as 3.19% <10%. In addition, R2 of Eqs (1) and (2) were 0.99849 and 0.93341, both higher than 0.90 (Figs 9 and 10). The above information indicated that cell growth kinetics model both described the cell dry weights and biomass concentrations constructed using Logistic equation can well explain the growth characteristics of R. erythropolis.
Figure 9.
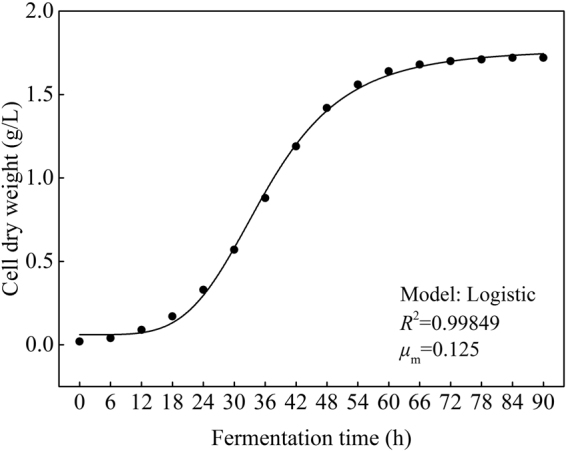
Fitting curve of cell growth kinetics which described the cell dry weights (Major experimental conditions: R. erythropolis was incubated in the potato starch wastewater medium of composition: 1 L potato starch wastewater, 4 g K2HPO4, 2 g KH2PO4, 0.2 g MgSO4, 0.1 g NaCl, and 2 g urea, with pH value of 6.8, at 150 r/min and 30 °C).
Figure 10.
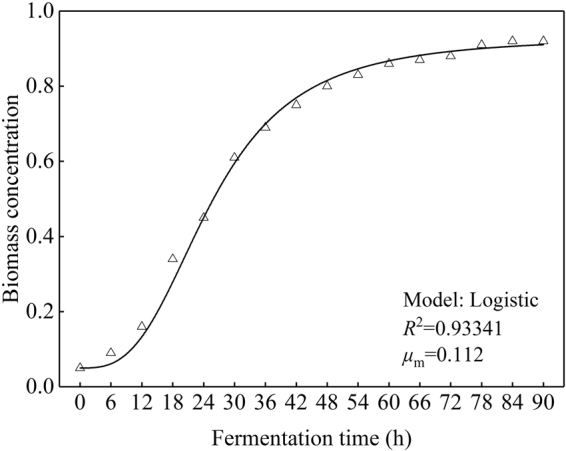
Fitting curve of cell growth kinetics which described the biomass concentrations (Major experimental conditions: R. erythropolis was incubated in the potato starch wastewater medium of composition: 1 L potato starch wastewater, 4 g K2HPO4, 2 g KH2PO4, 0.2 g MgSO4, 0.1 g NaCl, and 2 g urea, with pH value of 6.8, at 150 r/min and 30 °C).
Table 1.
Predicted and experimental values of the dry weight and biomass concentration.
| Fermentation time (h) | Cell dry weight (g/L) | Biomass concentration | ||||
|---|---|---|---|---|---|---|
| Experimental value | Predicted value | Relative error (%) | Experimental value | Predicted value | Relative error (%) | |
| 0 | 0.02 | 0.02 | 0 | 0.05 | 0.05 | 0 |
| 6 | 0.04 | 0.04 | 0 | 0.09 | 0.08 | 0 |
| 12 | 0.09 | 0.09 | 0 | 0.16 | 0.16 | 0 |
| 18 | 0.18 | 0.17 | 5.56 | 0.34 | 0.33 | 2.94 |
| 24 | 0.31 | 0.33 | 6.45 | 0.45 | 0.41 | 8.51 |
| 30 | 0.52 | 0.57 | 9.62 | 0.61 | 0.56 | 8.20 |
| 36 | 0.82 | 0.88 | 7.32 | 0.69 | 0.69 | 0 |
| 42 | 1.12 | 1.19 | 6.25 | 0.75 | 0.79 | 5.33 |
| 48 | 1.31 | 1.42 | 8.40 | 0.80 | 0.85 | 6.25 |
| 54 | 1.48 | 1.56 | 5.41 | 0.83 | 0.88 | 6.02 |
| 60 | 1.58 | 1.64 | 3.80 | 0.86 | 0.90 | 4.65 |
| 66 | 1.62 | 1.68 | 3.70 | 0.87 | 0.91 | 4.60 |
| 72 | 1.65 | 1.70 | 3.00 | 0.88 | 0.91 | 3.41 |
| 78 | 1.67 | 1.71 | 2.40 | 0.91 | 0.92 | 1.10 |
| 84 | 1.69 | 1.72 | 1.78 | 0.92 | 0.92 | 0 |
| 90 | 1.72 | 1.72 | 0 | 0.92 | 0.92 | 0 |
Bioflocculant synthesis kinetics
In this study, the bioflocculant production was almost simultaneously accompanied with cell growth, thus, the integration of Eq. (6) can be lead to:
| 3 |
Bioflocculant synthesis kinetics was obtained from the non-linear fit (Fig. 11) of Eq. (3) and given as follows:
| 4 |
From Table 2, the average relative error between the bioflocculant yields predicted by the kinetics model (predicted value) and measured from experiments (actual value) was appeared as 4.69% <10% when the strain was in the logarithm growth and stationary phases, and the R2 was appeared as 0.99880 >0.90 (Fig. 11), indicated that the bioflocculant synthesis kinetics model constructed using Luedeking-Piret equation can well explain the bioflocculant production process by R. erythropolis.
Figure 11.
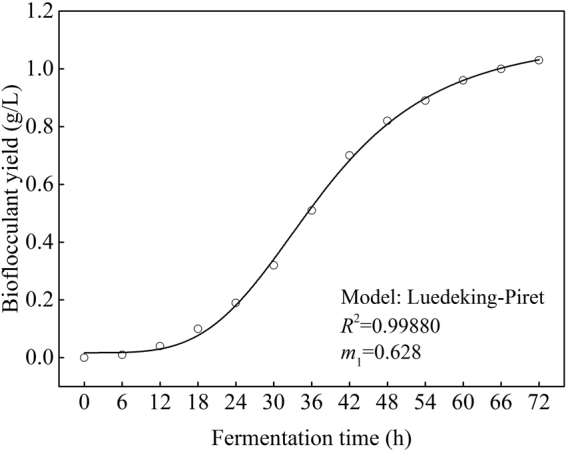
Fitting curve of bioflocculant synthesis kinetics (Major experimental conditions: R. erythropolis was incubated in the potato starch wastewater medium of composition: 1 L potato starch wastewater, 4 g K2HPO4, 2 g KH2PO4, 0.2 g MgSO4, 0.1 g NaCl, and 2 g urea, with pH value of 6.8, at 150 r/min and 30 °C).
Table 2.
Predicted and experimental values of the bioflocculant yields.
| Fermentation time (h) | Experimental value (g/L) | Predicted value (g/L) | Relative error (%) |
|---|---|---|---|
| 0 | 0 | 0 | 0 |
| 6 | 0.01 | 0.01 | 0 |
| 12 | 0.04 | 0.04 | 0 |
| 18 | 0.11 | 0.10 | 9.09 |
| 24 | 0.21 | 0.19 | 9.52 |
| 30 | 0.35 | 0.32 | 8.57 |
| 36 | 0.56 | 0.51 | 8.93 |
| 42 | 0.75 | 0.70 | 6.67 |
| 48 | 0.86 | 0.82 | 4.65 |
| 54 | 0.92 | 0.89 | 3.26 |
| 60 | 0.97 | 0.96 | 1.03 |
| 66 | 0.97 | 1.00 | 3.09 |
| 72 | 0.97 | 1.03 | 6.17 |
Preliminary application in pretreatment of potato starch wastewater
Figure 12 depicted COD removal at different bioflocculant doses and solution pH values. Under different solution pH values (4.5–11.5), COD removal rates achieved their maximum value at bioflocculant dose of 30 mg/L. In addition, the bioflocculant showed good performances in weak acid, weak alkaline and neutral pH conditions (6.5–9.5) for the removal of COD from potato starch wastewater. Especially, at solution pH point of 7.5, after treated by 30 mg/L of the bioflocculant, COD removal rate can reached 57.3%, which was decreased beyond this pH value. Similar conclusions for potato starch wastewater treatment by Paenibacillus polymyxa and its bioflocculant have been reported15.
Figure 12.
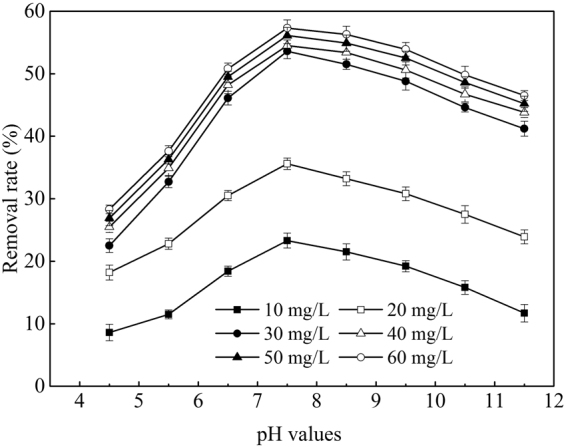
Effects of bioflocculant doses on COD removal under different pH values of the potato starch wastewater (Major experimental conditions: bioflocculant doses 10–60 mg/L, solution pH values 4.5–11.5).
Based on the good performance in pretreatment of potato starch wastewater in laboratory, real application scenario of the bioflocculant was investigated. Currently, in Ronghua Starch Co., Ltd., Sichuan Province, a sequencing batch reactor (SBR) was constructed to treat potato starch wastewater, due to the better anti-shock loading capability and system stability. Under the condition of influent average COD of 7836 mg/L, the temperature of 25 °C, continual aerations of 2.5 h and sludge density of 2000–3000 mg/L, the reactor had a good degeneration ability of COD and ammonium in potato starch wastewater, and the corresponding removal rates could reach 65% or more (without addition of bioflocculant) (Table 3). After treated under the same condition and at the same procedure with addition of 30 mg/L bioflocculant during a long period of 60 d, the average removal rates increased up to 97.3% for COD and 94.6% for ammonium.
Table 3.
Qualities of potato starch wastewater before and after treated by SBR (with or without bioflocculant addition) (under the condition of influent average COD of 7836 mg/L, temperature of 25 °C, continual aerations of 2.5 h and sludge density of 2000–3000 mg/L)
| Potato starch wastewater | After treated by SBR technology without bioflocculant | Removal rate (%) | After treated by SBR technology with 30 mg/L bioflocculant | Removal rate (%) | |
|---|---|---|---|---|---|
| COD (mg/L) | 7836 | 2492 | 68.2 | 211.6 | 97.3 |
| BOD5 (mg/L) | 2154 | 747.4 | 65.3 | 176.6 | 91.8 |
| Ammonium | |||||
| (mg/L) | 25.6 | 8.7 | 66.1 | 1.4 | 94.6 |
| TP | |||||
| (mg/L) | 18.3 | 7.2 | 60.8 | 0.27 | 98.5 |
| Turbidity (NTU) | 685 | 187.7 | 72.6 | 38.4 | 93.4 |
| Chroma | 45 | 13.3 | 70.5 | 2.7 | 94.0 |
| pH value | 6.8 | 6.5 | — | 6.6 | — |
Discussion
A bioflocculant was harvested by culturing R. erythropolis in potato starch wastewater medium, the optimum medium composition and the main backbone of this bioflocculant were similar with previous reports from this lab, in which Paenibacillus polymyxa was selected as the bioflocculant-producing strain15. The bioflocculant yield of 0.97 g/L was higher than that reported in similar previous researches14,22. How about this yield compared to that using normal medium? Under the same fermentation process in standard fermentation medium as peptone 10 g, beef extracts 3 g, K2HPO4 4 g, KH2PO4 2 g, MgSO4 2 g, and NaCl 5 g dissolved in 1 L distilled water with the pH value of 6.8, 1.12 g of bioflocculant can be extracted from 1 L of fermentation liquor after fermentation for 60 h, an increasing of about 15.5%, compared with the 0.97 g/L. This result suggested that the potato starch wastewater could be an effective alternative medium to produce bioflocculant.
Growth curve of R. erythropolis showed that the bioflocculant formation was almost simultaneously accompanied with cell growth, similar with the fermentation of Aspergillus parasiticus18. Cell growth and bioflocculant production could be simulated with both Logistic and Luedeking-Piret equations pretty well. COD, ammonium, and TP of the potato starch wastewater medium were rapidly down to 1736, 188, and 146 mg/L, respectively, while the exactly pH value was almost no change. The decline of COD, ammonium, and TP may be due to the fast utilization rate of organic matter and nitrogen by R. erythropolis in logarithm growth phase. Ammonium recovered slowly after the strain entered into death phase, in this phase, cell death and autolysis resulting in the releasing of intracellular protein into the wastewater medium17.
Further, from a practical standpoint, at natural pH value of the potato starch wastewater of 6.8, after treated by 30 mg/L bioflocculant, the residual COD, BOD5, ammonium, TP, turbidity, and chroma of the potato wastewater were detected as 3652.6, 883.4, 10.2, 8.5 mg/L, 247 NTU, and 15.7 times, which were decreased a lot compared with the initial concentrations (7836, 2154, 25.6, 18.3 mg/L, 685 NTU, 45 times), indicated that the bioflocculant flocculation can be used as an effective pretreatment way for the potato starch wastewater. The improvement of removal rate of COD from 68.2% to 97.3% and ammonium from 66.1% to 94.6% when the 30 mg/L of bioflocculant was applied to combine with the sequencing batch reactor (SBR), indicated that the bioflocculant was feasible in actual projects of potato starch wastewater treatment in Ronghua Starch Co., Ltd., Sichuan Province. Some other bacteria were also effective in pollutants removal from wastewaters, for example, Coelastrella sp. QY01 can remove 90% of ammonium and 90% of TP from aerobically treated swine wastewater after cultivated for 10 days23; Rhodobacter blasticus and Rhodobacter capsulatus can remove 83.3% of COD from aerobically treated swine wastewater after cultivated for 72 h24.
Materials and Methods
Potato starch wastewater
Potato starch wastewater used in this study was taken from Ronghua Starch Co., Ltd., Sichuan Province, and its water quality was shown in Table 4.
Table 4.
Qualities of potato starch wastewater and potato starch wastewater medium.
| Potato starch wastewater | Potato starch wastewater medium | |
|---|---|---|
| COD (mg/L) | 7836 | 7836 |
| BOD5 (mg/L) | 2154 | 2154 |
| Ammonium (mg/L) | 25.6 | 975 |
| Total phosphorous (mg/L) | 18.3 | 732 |
| Turbidity (NTU) | 685 | — |
| Chroma | 45 | 38 |
| pH value | 6.8 | 6.8 |
Bioflocculant production
R. erythropolis, deposited in China Center for Type Culture Collection (No. 10543), was specified to produce bioflocculant. Before bioflocculant production, R. erythropolis was first inoculated in 150 mL seed medium consisted of peptone 10 g, beef extracts 3 g, and NaCl 5 g dissolved in 1 L distilled water, and incubated on a reciprocal shaker (SHA-A, Shanghai Lianhua Company, China) at 150 r/min and 30 °C for 24 h. After cultivation, 2.0% v/v of the above inoculums was used to inoculate the potato starch wastewater medium of composition: 1 L potato starch wastewater, 4 g K2HPO4, 2 g KH2PO4, 0.2 g MgSO4, 0.1 g NaCl, and 2 g urea, and incubated in the same procedure to produce bioflocculant. Main characteristics of the potato starch wastewater medium was also shown in Table 4.
After fermentation for 60 h, the fermentation liquor was obtained, from the whole of which the bioflocculant was extracted by using the methods proposed by Guo et al.11, with the following modifications: the fermentation liquor with flocculating components was centrifuged at 3000 r/min for 30 min to obtain supernatant containing slime bioflocculant and pellet containing capsular bioflocculant. To determine the slime bioflocculant, the supernatant was precipitated with 2 volumes of chilled absolute acetone (containing 0.07% β-mercaptoethanol) by incubating the mixture at −20 °C for 4 h, then, the resulting precipitate was collected by centrifugation at 3000 r/min for 30 min and the slime bioflocculant was obtained. To determine the capsular bioflocculant, the pellet was re-suspended in phosphate solution, and heated at 40 °C for 15 min followed by centrifugation at 3000 r/min for 30 min, then, the supernatant was treated in the same the same procedure as the determination of slime bioflocculant to obtain the capsular bioflocculant. Sum of the slime and the capsular was denoted as the bioflocculant produced by Rhodococcus erythropolis in this study.
Physical and chemical analysis of the bioflocculant
Total sugar content of the purified bioflocculant was determined by the phenol-sulfuric acid method25. Protein content was measured by the Bradford method26. Molar mass was determined by gel permeation chromatography (GPC) using a Hitachi L-6200 system controller. Functional groups were determined by using a Fourier transform infrared spectrophotometer (EQUINOX 55, Bruker Company, Germany). Thermal stability of the bioflocculant was determined as follows: the bioflocculant was dissolved in a suitable volume of deionized water to achieve an initial flocculating activity of over 90% and divided into 12 aliquots, which were placed at 10, 20, 30, 40, 50, 60, 70, 80, 90, 100, 110, and 120 °C for 30 min, respectively. Subsequently, their flocculating activities were measured. The thermal stability was judged according to the changeable of the flocculating activities.
Assay of flocculating activity
Flocculating activity was measured in jar tests referenced to the method reported in our previous publication15, in which 4 g/L of kaolin clay suspension was chosen as the suspended solid, whose optical density (OD) were measured with a spectrophotometer (Unic–7230, Shanghai Lianhua Company, China) at 550 nm before and after treated by the bioflocculant. The control experiment was conducted in the same manner without adding bioflocculant.
Determination of biomass concentration, colonies number, and cell dry weight
Biomass concentration was measured by turbidimetry method using a spectrophotometer (Unic–7230, Shanghai Lianhua Company, China) at 600 nm, numbers of colonies were counted using the plate count method17. Cell dry weight was determined as follows: the fermentation liquor at different fermentation time was centrifuged at 3000 r/min for 10 min, and the resulting precipitate (strain cell) was collected and then dried in an oven at 105 °C until a constant weight, which was denoted as cell dry weight.
Determination of COD, ammonium, and TP
COD, ammonium, and TP of the potato starch wastewater medium were determined according to the EPA Standard Methods27.
Fermentation kinetics
Generally, Logistic equation could nicely describe the dynamic process of cell growth, and was normally given as follows:
| 5 |
where X0 and Xm were the initial and the maximum cell dry weight (or biomass concentration), respectively; X(t) was the cell dry weight (or biomass concentration) at fermentation time t (g/L); μm was a constant that characterized the maximum specific growth rate (h−1); t was the fermentation time (h).
Luedeking-Piret equation was always applied to simulate the process of bioflocculant production, and was normally given as follows:
| 6 |
Where P was the bioflocculant yield (g/L); m1 and m2 were the equation parameters, they were constants when the fermentation conditions were determined.
Actual application
Potato starch wastewater from Ronghua Starch Co., Ltd., Sichuan Province was selected to identify the potential of the bioflocculant in actual application. Bioflocculant dose and pH values of the potato starch wastewater were used to optimize the flocculating conditions. A sample of 1.0 L wastewater was poured in a beaker and the pH value was adjusted using 1.0 mol/L NaOH or HCl if necessary. The bioflocculant was then added, and the mixture was stirred at the design agitation speed for 10 min, and then allowed to stand 30 min. Referenced to the treatment process reported in our previous publication15, the residual COD, BOD5, ammonium, TP, turbidity, and chroma of the potato wastewater were determined after the pretreatment.
All the measurements through this study were carried out in triplicates and the average values were presented (with standard error less than 5% of the mean).
Electronic supplementary material
Acknowledgements
Authors would like to acknowledge the finding support for this study by the National Natural Science Foundation of China (Grant No. 51508043, 51608061) and Basic Project of Science and Technology Department of Sichuan Provincial (2016JY0015, 2015JY0113, 2014JY0245).
Author Contributions
J.G. conceived the project and contributed to the conception of the study and wrote the paper. J.L., Y.Y., Y.Z., S.J., and C.C. performed experiments and data analysis. All authors approved the final version of the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-21796-x.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Liu W, Yuan H, Yang J, Li B. Characterization of bioflocculants from biologically aerated filter backwashed sludge and its application in dying wastewater treatment. Bioresource Technol. 2009;100:2629–32. doi: 10.1016/j.biortech.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 2.Liu X, et al. Landfill leachate pretreatment by coagulation-flocculation process using iron-based coagulants: Optimization by response surface methodology. Chem Eng J. 2012;200:39–51. doi: 10.1016/j.cej.2012.06.012. [DOI] [Google Scholar]
- 3.Guo J, et al. Enhanced efficiencies of sludge dewatering and domestic wastewater treatment by using the bioflocculant from rice stover. Water and Environ J. 2017;31:120–26. doi: 10.1111/wej.12221. [DOI] [Google Scholar]
- 4.Campbell, A. The Potential role of aluminum in Alzheimer’s disease. 17(Suppl 2), 17—20 (2002). [DOI] [PubMed]
- 5.Rudén C. Acrylamide and cancer risk–expert risk assessments and the public debate. Food Chem Toxicol. 2004;42:335–49. doi: 10.1016/j.fct.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 6.Li Z, et al. Production of a novel bioflocculant by Bacillus licheniformis X14 and its application to low temperature drinking water treatment. Bioresource Technol. 2009;100:3650–6. doi: 10.1016/j.biortech.2009.02.029. [DOI] [PubMed] [Google Scholar]
- 7.Guo J. Characteristics and mechanisms of Cu(II) sorption from aqueous solution by using bioflocculant MBFR10543. Appl Microbiol Biot. 2015;99:229–40. doi: 10.1007/s00253-014-6103-y. [DOI] [PubMed] [Google Scholar]
- 8.Liu J, et al. Research on microbial lipid production from potato starch wastewater as culture medium by Lipomycesstarkeyi. Water Sci Technol. 2013;67:1802–8. doi: 10.2166/wst.2013.059. [DOI] [PubMed] [Google Scholar]
- 9.Wang L, et al. Bioflocculants from hydrolysates of corn stover using isolated strain Ochrobactium ciceri W2. Bioresource Technol. 2013;145:259–63. doi: 10.1016/j.biortech.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 10.Li Y, et al. Characterization and flocculation mechanism of an alkali-activated polysaccharide flocculant from Arthrobacter sp. B4. Bioresource Technol. 2014;170:574–7. doi: 10.1016/j.biortech.2014.07.112. [DOI] [PubMed] [Google Scholar]
- 11.Guo J, Yang C, Peng L. Preparation and characteristics of bacterial polymer using pre-treated sludge from swine wastewater treatment plant. Bioresource Technol. 2014;152:490–8. doi: 10.1016/j.biortech.2013.11.037. [DOI] [PubMed] [Google Scholar]
- 12.Wang S, et al. Production of a novel bioflocculant by culture of Klebsiella mobilis using dairy wastewater. Biochem Eng J. 2007;36:81–6. doi: 10.1016/j.bej.2007.02.003. [DOI] [Google Scholar]
- 13.More T, Yan S, Tyagi RD, Surampalli RD. Potential use of filamentous fungi for wastewater sludge treatment. Bioresource Technol. 2010;101:7691–700. doi: 10.1016/j.biortech.2010.05.033. [DOI] [PubMed] [Google Scholar]
- 14.Pu S, et al. Preparation and application of a novel bioflocculant by two strains of Rhizopus sp. using potato starch wastewater as nutrilite. Bioresource Technol. 2014;162:184–91. doi: 10.1016/j.biortech.2014.03.124. [DOI] [PubMed] [Google Scholar]
- 15.Guo J, Lau AK, Zhang Y, Zhao J. Characterization and flocculation mechanism of a bioflocculant from potato starch wastewater. Appl Microbiol Biot. 2015;99:5855–61. doi: 10.1007/s00253-015-6589-y. [DOI] [PubMed] [Google Scholar]
- 16.Xia S, et al. Production and characterization of a bioflocculant by Proteus mirabilis TJ-1. Bioresource Technol. 2008;99:6520–7. doi: 10.1016/j.biortech.2007.11.031. [DOI] [PubMed] [Google Scholar]
- 17.Guo J, Xin X, Nengzi L, Li J. Fermentation and kinetics characteristics of bioflocculant production using swine wastes as raw material. China Environ Sci. 2014;34:2588–92. [Google Scholar]
- 18.Deng S, Yu G, Ting Y. Production of a bioflocculant by Aspergillus parasiticus and its application in dye removal. Colloids Surf B: Biointerfaces. 2005;44:179–86. doi: 10.1016/j.colsurfb.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 19.Peng L, et al. Characterization and application of bioflocculant prepared by Rhodococcus erythropolis using sludge and livestock wastewater as cheap culture media. Appl Microbiol Biot. 2014;98:6847–58. doi: 10.1007/s00253-014-5725-4. [DOI] [PubMed] [Google Scholar]
- 20.Giri S, et al. Production and characterization of a thermostable bioflocculant from Bacillus subtilis F9, isolated from wastewater sludge. Ecotox Environ Safe. 2015;121:45–50. doi: 10.1016/j.ecoenv.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 21.Sun J, Zhang X, Miao X, Zhou J. Preparation and characteristics of bioflocculants from excess biological sludge. Bioresource Technol. 2012;126:362–6. doi: 10.1016/j.biortech.2012.08.042. [DOI] [PubMed] [Google Scholar]
- 22.Ahmad H, Idris A, Abdullah N, Mohamad R. Production and characterization of a bioflocculant produced by Aspergillus flavus. Bioresource Technol. 2013;127:489–93. doi: 10.1016/j.biortech.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 23.Luo L, et al. Nutrient removal and lipid production by Coelastrella sp. in anaerobically and aerobically treated swine wastewater. Bioresource Technol. 2016;216:135–41. doi: 10.1016/j.biortech.2016.05.059. [DOI] [PubMed] [Google Scholar]
- 24.Wen S, et al. Treatment of anaerobically digested swine wastewater by Rhodobacter blasticus and Rhodobacter capsulatus. Bioresource Technol. 2016;222:33–38. doi: 10.1016/j.biortech.2016.09.102. [DOI] [PubMed] [Google Scholar]
- 25.Chaplin M, Kennedy J. Carbohydrate analysis, seconded. New York: Oxford University Press; 1994. [Google Scholar]
- 26.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 27.EPA of China. Water and waste water monitoring methods (in Chinese). (China Environmental Science Press. Beijing, 2002).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


