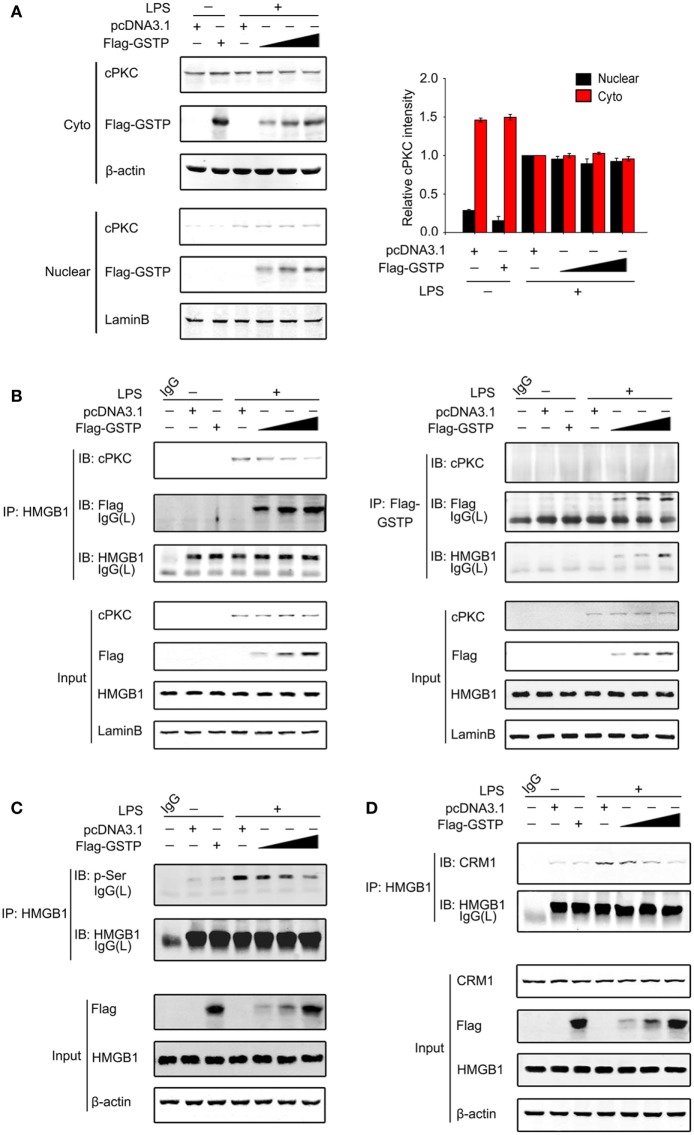
Figure 7.
Nuclear glutathione S-transferase Pi (GSTP) inhibits the interaction between classic protein kinase C (cPKC) and high mobility group box-1 protein (HMGB1). (A,B) Flag-GSTP (0.5, 1, or 2 µg) or empty vector was transfected into RAW264.7 cells. After 36 h, cells were incubated with lypopolysaccharide (LPS; 500 ng/ml) for 20 min. (A) Nuclear and cytoplasmic fractions were subjected to immunoblot analysis with anti-cPKC or anti-Flag antibody. (B) Nuclear extracts were subjected to immunoprecipitation with anti-HMGB1 or anti-Flag antibody and then were immunoblot analyzed with anti-Flag and anti-HMGB1 antibodies. Whole nuclear lysates were immunoblot analyzed by using anti-cPKC, anti-Flag, anti-HMGB1, or anti-lamin B antibody, respectively. (C,D) Flag-GSTP (0.5, 1, or 2 µg) or empty vector was transfected into RAW264.7 cells. (C) Cells were incubated with LPS (500 ng/ml) for 4 h. Cell lysates were immunoprecipitated using HMGB1 antibody and analyzed by immunoblotting with p-Ser antibody. Whole cell lysates were subjected to immunoblotting with Flag-GSTP, HMGB1 and β-actin antibodies. (D) Cells were treated with LPS (500 ng/ml) for 6 h. Cell lysates were immunoprecipitated with anti-HMGB1 antibody and analyzed by immunoblotting using HMGB1 and chromosomal region maintenance 1 antibodies. The blots were representative of three independent experiments.