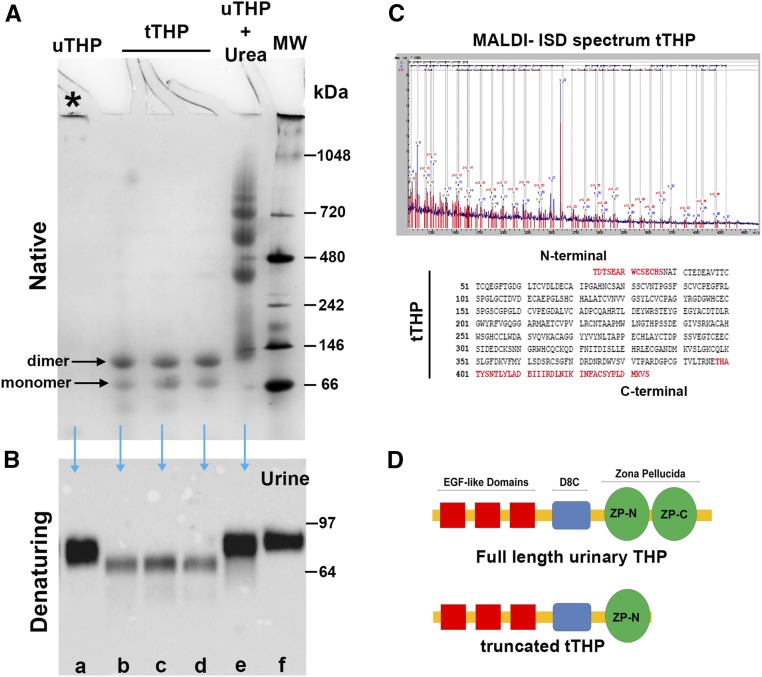
Figure 1.
Characterization of urinary THP. (A) Electrophoresis under native conditions of various forms of THP: urinary THP purified by the method of Tamm and Horsfall (uTHP, lane a), truncated THP isolated by size exclusion chromatography (tTHP, lanes b–d), and urinary THP treated with 8 M urea (lane e). Molecular mass markers are shown in lane f. Aggregated uTHP does not undergo electrophoresis under native conditions (asterisk). Two bands of tTHP corresponding to a monomer and a dimer are observed. Multiple high–molecular mass aggregates are observed in lane e, reflecting the chaotropic effect of urea on THP multimers. (B) Western blotting of SDS-PAGE applied on to the same forms of THP is seen, with the addition of a urine sample in lane f. tTHP is now reduced to a single band, which is in the range of 64–68 kD. (C) Proteomic characterization of tTHP using MALDI-ISD maps the C-terminal sequence of tTHP to a region ending in amino acid 434, which is in the region within the two subdomains (ZP-N and ZP-C) of the ZP, with an estimated molecular mass around 65 kD. (D) Schematic representation of full-length THP and tTHP, underscoring the site of truncation at the ZP domain. MALDI-ISD, matrix-assisted laser desorption ionization-in source decay.