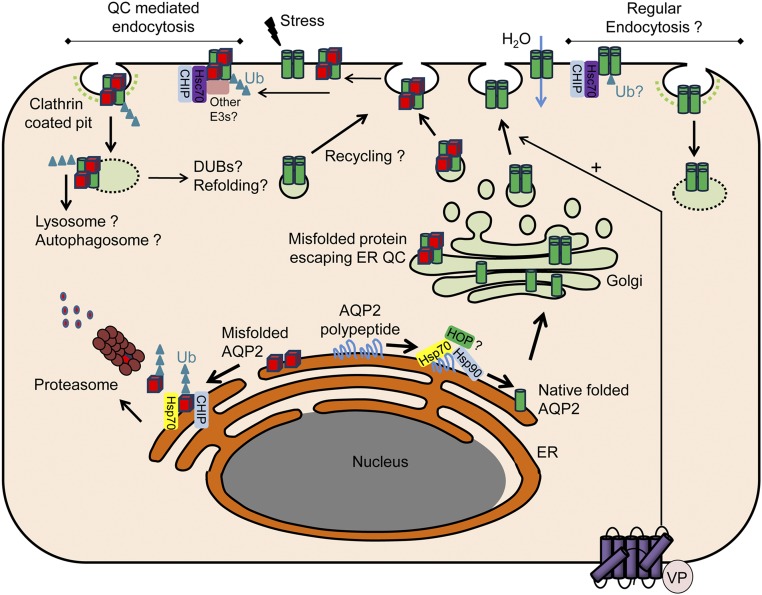
Figure 11.
Potential role of CHIP in AQP2 QC and trafficking. In the endoplasmic reticulum (ER) under normal physiologic conditions, AQP2 polypeptide is bound by the chaperone protein Hsp70. The cochaperone Hop facilitates the association with the Hsp90 complex, promoting native folding of AQP2 that traffics to the Golgi apparatus, is organized into tetramers, and stored in transport vesicles. As part of the ER QC, Hsp70-bound AQP2 that is misfolded under normal conditions or after cellular stress associates with CHIP and is targeted to endoplasmic reticulum–associated degradation (ERAD) via the ubiquitin proteasome system. Other ERAD-associated E3 ligases are likely to be involved in ubiquitylation of AQP2. Despite ER QC, a proportion of AQP2 escapes to the Golgi apparatus and is subsequently stored in transport vesicles. Folded and misfolded AQP2 traffics to the plasma membrane (PM) after AVP (VP) simulation. Because of peripheral QC, ER-misfolded AQP2 or AQP2 misfolded within the PM due to environmental stresses are recognized and bound by Hsc70. Hsc70 associates with CHIP and other E3 ligases promoting AQP2 endocytosis and degradation. Alternatively, internalized AQP2 may be deubiquitylated, refolded, and recycled to the PM. In addition to QC-mediated endocytosis, regular AQP2 endocytosis may involve Hsc70/CHIP. A number of the collecting duct–specific E3 ligases and deubiquitylases (DUBs) identified may play important roles in these processes. Ub, ubiquitin.