Abstract
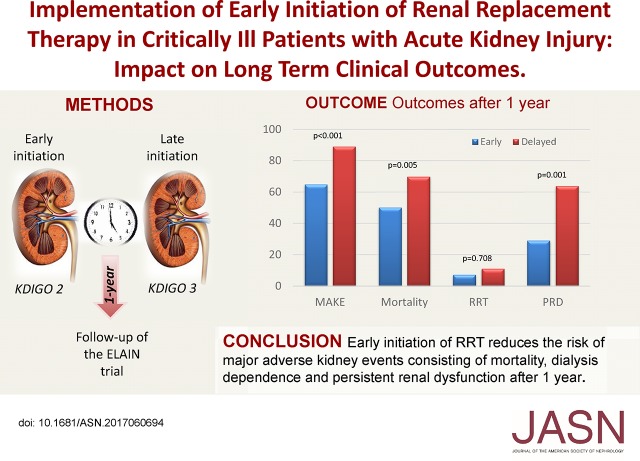
Whether earlier initiation of RRT in critically ill patients with AKI can improve outcomes remains debated. We examined follow-up data from a large clinical trial to prospectively investigate the long-term outcomes associated with the timing of RRT initiation in such patients. We extended the follow-up of patients in the Early Versus Delayed Initiation of RRT in Critically Ill Patients with AKI (ELAIN) Trial from 90 days to 1 year after randomization for 230 (99.6%) patients. The primary outcome was a composite of major adverse kidney events (persistent renal dysfunction, dialysis dependence, and mortality) at 1 year. Secondary outcomes included inflammatory markers. Overall, 72 of 111 (64.9%) and 106 of 119 (89.1%) patients met the primary outcome in the early (stage 2 AKI) and delayed (stage 3 AKI) initiation groups, respectively (odds ratio [OR] with early initiation, 0.23; 95% confidence interval [95% CI], 0.11 to 0.45; P< 0.001). The early initiation group had a 1-year all-cause mortality rate (56 of 111 [50.2%]) significantly lower than that of the delayed initiation group (83 of 119 [69.8%]; absolute difference, −19.6%; 95% CI, −32.0% to −7.2%; P<0.01). After 1 year, 16 of 55 (29.1%) and 23 of 36 (63.9%) surviving patients in the early and delayed groups, respectively, failed to recover renal function (absolute difference, −34.8%; 95% CI, −54.6% to −15.0%; P=0.001). In conclusion, early initiation of RRT in these critically ill patients with AKI significantly reduced the occurrence of major adverse kidney events, reduced mortality, and enhanced renal recovery at 1 year.
Keywords: acute renal failure, hemodialysis, survival
AKI is one of the most common complications in critically ill patients, occurring in approximately 50% of all patients in the intensive care unit.1 When severe enough to receive RRT, AKI is associated with hospital mortality rates ranging from 50% to >70%.2–5 Long-term outcomes of patients with AKI receiving RRT are less clear. Existing descriptions of these long-term outcomes have been obtained from retrospective cohorts, and they have used variable methodologies. Some of these reports have been on the basis of population cohorts,6,7 whereas others have been on the basis of specific disease groups.8–10 Another limitation of these AKI cohorts is that they are often determined by using post-AKI exposure data, such as hospitalization coding,10 or on the basis of survival of the acute hospitalization.7 For clinicians treating patients with AKI, the utility of the available data is subject to these limitations.
AKI is associated with numerous in-hospital and long-term adverse consequences, including increased risk of death.11,12 Although the increased mortality from AKI is well documented, the effect of an episode of AKI on other outcomes has only recently been brought into focus, particularly with regard to the increased risk of CKD.13,14 A large population cohort study in 2009 concluded that AKI necessitating in-hospital dialysis was associated with an increased risk of chronic dialysis but was not associated with an increase in all-cause mortality.6 In some cohorts of patients, up to 15% of patients with an episode of AKI remain dialysis dependent after hospital discharge.7 Moreover, patients surviving an episode of dialysis-dependent AKI are at risk for upper gastrointestinal bleeding,15 stroke,16 and cardiovascular events (myocardial injury and congestive heart failure).17,18
Approximately 5%–8% of all patients in the intensive care unit receive RRT, and these patients have the highest short-term mortality of any group with AKI.11 Studies that have examined the timing of RRT have shown conflicting results in short-term outcomes.19,20 Longer-term outcomes of patients in whom RRT was initiated at different time points (early versus delayed) are unknown.
We previously reported the results of a randomized, controlled trial comparing early versus delayed initiation of RRT in critically ill patients with AKI.20 We showed a significantly reduced 90-day all-cause mortality in the early group compared with the delayed group. However, the benefit of the intervention may not be sustained, and thus, the aim of this study was to extend follow-up to up to 1 year and report clinically relevant patient-centered outcomes (including persistent renal dysfunction, dialysis dependence, and mortality) in critically ill patients with AKI in whom RRT was started at different time points.
Results
Outcome Variables
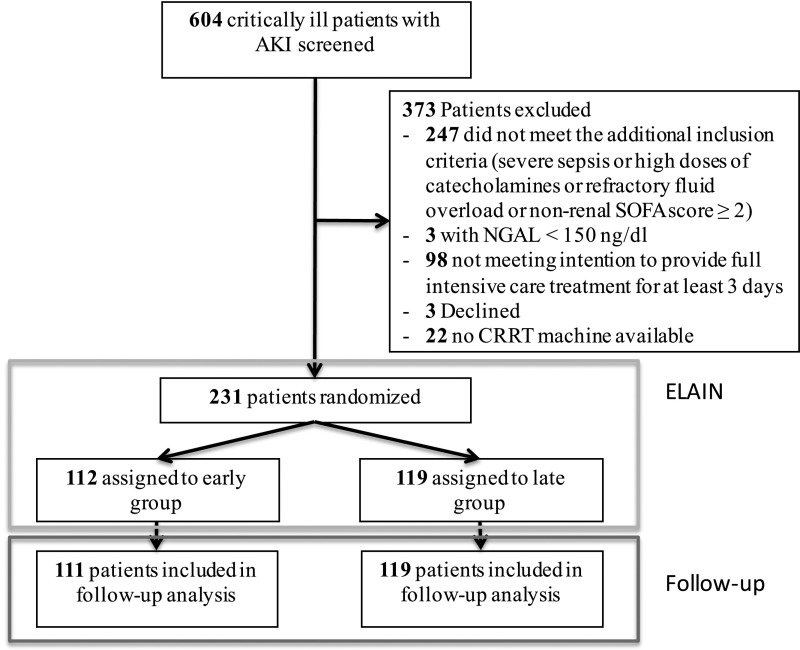
In total, 231 patients were enrolled in the Early Versus Delayed Initiation of RRT in Critically Ill Patients with AKI (ELAIN) Trial and randomized to receive either early (Kidney Disease Improving Global Outcomes [KDIGO] stage 2; n=112) or delayed (KDIGO stage 3; n=119) initiation of RRT. As previously shown, the baseline characteristics did not differ between the two groups.20 In this follow-up analysis, we evaluated 230 patients, because one patient was lost to follow-up in the early group (Figure 1).
Figure 1.
The flowchart of the ELAIN Trial shows 231 patients were included in the initial trial and 1 patient was lost to follow-up for the follow-up analysis. CRRT, continuous RRT; NGAL, neutrophil gelatinase–associated lipocalin.
In patients randomized to early initiation, significantly fewer patients met the combined end point major adverse kidney event at day 365 (MAKE365; consisting of death, RRT, and persistent renal dysfunction at 1 year) compared with patients randomized to delayed initiation (72 of 111 [64.9%] versus 106 of 119 [89.1%]; absolute difference, −24.2%; 95% confidence interval [95% CI], −34.7% to −13.7%; P<0.001) (Table 1).
Table 1.
Outcomes of patients by treatment group
| Outcome Parameter | Early, n=112 | Delayed, n=119 | P Value | Absolute Difference Early − Delayed [95% CI], % | OR, Early Versus Delayed [95% CI] |
|---|---|---|---|---|---|
| Primary outcome | |||||
| MAKE 1 yr,a no./no. total (%) | 72/111 (64.9) | 106/119 (89.1) | <0.001 | −24.2 [−34.7 to −13.7] | 0.23 [0.11 to 0.45] |
| Secondary outcomes | |||||
| 1-yr all-cause mortality, no. (%) | 56/111 (50.2) | 83/119 (69.8) | <0.01 | −19.6 [−32.0 to −7.2] | 0.62 [0.44 to 0.87] |
| Requirement of RRT 1 yr, no./no. total (%) | 4/55 (7.3) | 4/36 (11.1) | 0.71 | −3.8 [−16.2 to 8.5] | 0.62 [0.15 to 2.69] |
| PRD 1 yr,b no./no. total (%) | 16/55 (29.1) | 23/36 (63.9) | 0.001 | −34.8 [−54.6 to −15.0] | 0.23 [0.09 to 0.57] |
MAKE is defined as mortality at 1 yr, RRT at 1 yr, and PRD defined as 25% decline in eGFR.
PRD defined as 25% decline in eGFR.
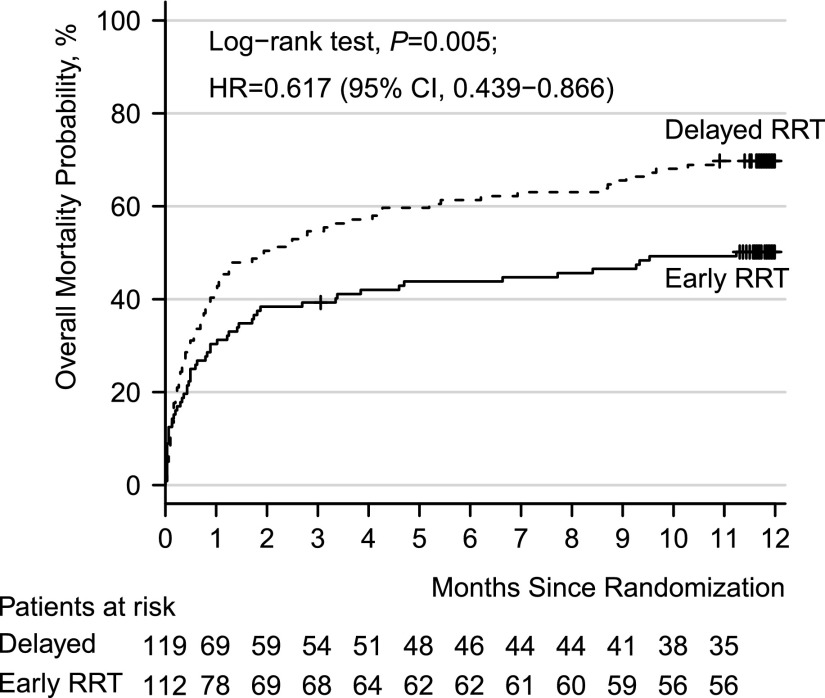
By analyzing the individual components of the combined end point, we showed that early initiation of RRT significantly reduced all-cause mortality at 1 year (56 of 111 [50.2%] versus 83 of 119 [69.8%]; absolute difference, −19.6%; 95% CI, −32.0% to −7.2%; P<0.01) (Figure 2, Table 1). Of the 139 patients not surviving to 1 year, significantly fewer patients died of multiple organ dysfunction syndrome in the early (12 of 56 [21.4%]) compared with the delayed group (32 of 83 [38.6%]; P=0.03). There were no differences regarding other causes of mortality (Supplemental Table 1). Sensitivity analyses of overall survival showed that the positive effects of early RRT do not seem to be restricted to the early follow-up phase but do seem to be maintained during the later follow-up phase (hazard ratio [HR], 0.49; 95% CI, 0.24 to 1.02; P=0.05) (Supplemental Figure 1). The HRs of early versus delayed RRT during the early follow-up phase up to day 90 and during late follow-up beyond day 90 were not significantly different (P=0.49): HR of early versus delayed RRT during the early follow-up phase up to day 90, 0.66; 95% CI, 0.45 to 0.97 (P=0.03) and HR of early versus delayed RRT during late follow-up beyond day 90, 0.49; 95% CI, 0.24 to 1.02 (P=0.06).
Figure 2.
The Kaplan Meier analysis shows the overall mortality in the early versus the delayed RRT initiation group. Patients with early initiation of RRT showed a significant improved survival as compared with the delayed group (HR, 0.617; 95% CI, 0.439–0.866; P=0.005).
Furthermore, in those patients who survived the 1-year follow-up, early initiation of RRT significantly reduced persistent renal dysfunction (16 of 55 [29.1%] in the early versus 23 of 36 [63.9%] in the delayed group; absolute difference, −34.8%; 95% CI, −54.6% to −15.0%; P=0.001) but did not reduce requirement of RRT at 1 year (four of 55 [7.3%] in the early versus four of 36 [11.1%] in the delayed group; absolute difference, −3.8%; 95% CI, −16.2% to 8.5%; P=0.71).
Of the 119 patients randomized to the delayed group, six patients did not progress to KDIGO stage 3. In the early phase of the trial (within 90 days), three patients died before progressing to KDIGO stage 3, and three patients recovered spontaneously. Of these three spontaneous recovered patients, two died within 1 year.
In multivariable logistic regression analysis, early initiation was associated with reduced risk for MAKE365 (odds ratio [OR], 0.23; 95% CI, 0.12 to 0.47; P<0.001), whereas an increased age was an independent risk factor for MAKE365 (OR, 1.34; 95% CI, 1.06 to 1.70; P=0.02) (Table 2). An increased age and Sequential Organ Failure Assessment (SOFA) score were independent risk factors for 1-year all-cause mortality, whereas early initiation in young patients (age <80 years old) was associated with reduced 1-year all-cause mortality (Table 3). More patients in the group >80 years old suffered from cardiac arrhythmias (P=0.06), valvular disease (P=0.05), and arterial hypertension (P=0.06) (Supplemental Table 2).
Table 2.
Predictors of MAKE365 by clinical factors (multivariable logistic regression)
| MAKE365 by Clinical Factors | OR | 95% CI | P Value |
|---|---|---|---|
| Randomization (early versus delayed) | 0.23 | 0.12 to 0.47 | <0.001 |
| Age at randomization, +10 yr | 1.34 | 1.06 to 1.70 | 0.02 |
Table 3.
Predictors of 1-year all-cause mortality by clinical factors (multivariable Cox regression)
| 1-yr All-Cause Mortality by Clinical Factors | HR | 95% CI | P Value |
|---|---|---|---|
| Patients ≤80 yr of age, n=206: randomization (early versus delayed) | 0.58 | 0.40 to 0.83 | 0.003 |
| Patients >80 yr of age, n=25: randomization (early versus delayed) | 1.85 | 0.89 to 3.84 | 0.10 |
| Age at randomization, +10 yr | 1.21 | 1.04 to 1.41 | 0.01 |
| SOFA 3–4 and/or oliguric, n=139 versus SOFA 0–2 and/or normuric, n=92 | 1.86 | 1.30 to 2.67 | <0.001 |
Furthermore, elevated log-transformed IL-8 (HR, 1.27; 95% CI, 1.11 to 1.45; P<0.001), IL-6 (HR, 1.19; 95% CI, 1.05 to 1.35; P<0.01), MIF (HR, 1.23; 95% CI, 1.06 to 1.43; P<0.01), IL-10 (HR, 1.39; 95% CI, 1.22 to 1.59; P<0.001), and TNFR-1 (HR, 1.38; 95% CI, 1.06 to 1.81; P=0.02) levels on day 3 after randomization were significant predictors of 1-year all-cause mortality (Table 4).
Table 4.
Predictors of 1-yr all-cause mortality by clinical factors and log-scale pro- and anti-inflammatory mediators (multivariable Cox regression)
| 1-yr All-Cause Mortality by Clinical Factors and Pro- and Anti-Inflammatory Markers | HR | 95% CI | P Value |
|---|---|---|---|
| Log(MIF), d3, +1 unit | 1.23 | 1.06 to 1.43 | <0.01 |
| Log(IL-6), d3, +1 unit | 1.19 | 1.05 to 1.35 | <0.01 |
| Log(IL-8), d3, +1 unit | 1.27 | 1.11 to 1.45 | <0.001 |
| Log(IL-10), d3, +1 unit | 1.39 | 1.22 to 1.59 | <0.001 |
| Log(IL-18), d3, +1 unit | 1.07 | 0.92 to 1.23 | 0.39 |
| Log(TNFR-1), d3, +1 unit | 1.38 | 1.06 to 1.81 | 0.02 |
| Log(TNFR-2), d3, +1 unit | 1.12 | 0.86 to 1.45 | 0.41 |
d3, Blood samples were drawn 72 h after randomization.
Exploratory Biomarker Analyses
An average of 159 of 204 (77.9%) had biomarkers measured from the blood collected on day 3 after randomization (Supplemental Table 3). Pro- (MIF, IL-6, IL-8, and IL-18) and anti-inflammatory (IL-10) biomarkers were measured in the blood at different time points. At the time of randomization, MIF, IL-6, IL-8, IL-10, and IL-18 did not differ between patients who met or did not meet MAKE365 (Table 5). Patients who met MAKE365 had significantly higher plasma concentrations of TNFR-1 and TNFR-2 72 hours after randomization (TNFR-1: MAKE365+: 2023.4 pg/ml [quartile 1 (Q1), Q3: 1200.5, 2995.0 pg/ml] and MAKE365−: 1447.8 pg/ml [Q1, Q3; 951.1, 2021.1 pg/ml]; P=0.01; TNFR-2: MAKE365+: 24,820.0 pg/ml [Q1, Q3; 16,775.5, 39,677.5 pg/ml] and MAKE365−: 20,880.0 pg/ml [Q1, Q3; 11,916.3, 29,420.0 pg/ml]; P=0.04) (Table 5). Plasma concentrations of MIF, IL-6, IL-8, IL-10, and IL-18 were not different (Table 5).
Table 5.
Analysis of pro- and anti-inflammatory markers (MAKE365)
| Pro- and Anti-Inflammatory Markers | Patients without MAKE365, d0 | Patients with MAKE365, d0 | P Value | Patients without MAKE365, d3 | Patients with MAKE365, d3 | P Value |
|---|---|---|---|---|---|---|
| MIF, median (Q1, Q3), pg/ml | 15,019.2 (10,189.2, 44,648.4) | 18,870.6 (9500.1, 45,180.3) | 0.96 | 11,283.6 (5988.6, 23,681.1) | 95,32.2 (4881.6, 19,211.7) | 0.24 |
| IL-6, median (Q1, Q3), pg/ml | 11,51.4 (354.1, 1794.9) | 967.4 (321.8, 2102.5) | 0.60 | 282.7 (102.1, 552.1) | 253.7 (95.4, 687.4) | 0.81 |
| IL-8, median (Q1, Q3), pg/ml | 313.6 (123.5, 571.2) | 256.6 (92.5, 538.5) | 0.73 | 73.3 (30.9, 129.2) | 47.3 (25.5, 120.1) | 0.29 |
| IL-10, median (Q1, Q3), pg/ml | 44.6 (20.8, 153.0) | 47.4 (18.3, 203.8) | 0.85 | 22.2 (9.6, 48.6) | 16.8 (9.7, 52.9) | 0.91 |
| IL-18, median (Q1, Q3), pg/ml | 727.0 (343.7, 1153.7) | 575.5 (285.0, 1376.7) | 0.63 | 777.3 (350.9, 1849.6) | 539.8 (301.8, 1448.0) | 0.14 |
| TNFR-1, median (Q1, Q3), pg/ml | 1600.3 (1221.1, 2104.9) | 1788.2 (1122.2, 2521.0) | 0.50 | 1447.8 (951.1, 2021.1) | 2023.4 (1200.5, 2995.0) | <0.01 |
| TNFR-2, median (Q1, Q3), pg/ml | 22,630.0 (14,873.8, 27,055.0) | 18,901.6 (12,856.0, 28,880.0) | 0.23 | 20,880.0 (11,916.3, 29,420.0) | 24,820.0 (16,775.5, 39,677.5) | <0.04 |
d0, Blood samples were drawn at the time of randomization; d3, blood samples were drawn 72 h after randomization.
To investigate whether biomarker associations vary between the different components of MAKE365, we analyzed the different components. Patients who died within the first year after randomization had significantly higher plasma concentrations of TNFR-1 and TNFR-2 3 days after randomization compared with patients surviving this time period (TNFR-1: 1558.6 pg/ml in survivors versus 2019.4 pg/ml in nonsurvivors; P=0.03; TNFR-2: 20,220.0 pg/ml in survivors versus 25,290.0 pg/ml in nonsurvivors; P=0.03) (Table 6). Patients who were still dialysis dependent at 1 year showed no significant changes in biomarker levels compared with patients not on dialysis (Table 7). However, the patient cohort was very small (eight patients in total). Patients who had a PRD at 1 year showed significant lower levels of MIF and IL-18 on day 3 (MIF: 6588.8 pg/ml in patients with PRD versus 11283.6 pg/ml in patients without PRD; P=0.04; IL-18: 381.2 pg/ml in patients with PRD versus 777.3 pg/ml in patients without PRD; P<0.01) compared with patients who did not have PRD at 1 year (Table 8).
Table 6.
Analysis of pro- and anti-inflammatory markers (mortality)
| Pro- and Anti-Inflammatory Markers | Patients Alive at 1 yr, d0 | Patients Dead at 1 yr, d0 | P Value | Patients Alive at 1 yr, d3 | Patients Dead at 1 yr, d3 | P Value |
|---|---|---|---|---|---|---|
| MIF, median (Q1, Q3), pg/ml | 16,227.0 (9778.2, 42,386.4) | 19,912.8 (9759.6, 46,549.8) | 0.81 | 10,730.4 (4699.8, 16,597.2) | 9715.2 (5549.4, 24,394.2) | 0.63 |
| IL-6, median (Q1, Q3), pg/ml | 982.5 (319.4, 2010.6) | 985.8 (329.4, 2033.5) | 0.92 | 261.4 (100.9, 670.4) | 260.9 (89.7, 647.9) | 0.85 |
| IL-8, median (Q1, Q3), pg/ml | 255.2 (94.1, 538.5) | 315.0 (94.8, 543.8) | 0.43 | 61.7 (27.0, 110.6) | 46.0 (26.8, 125.7) | 0.90 |
| IL-10, median (Q1, Q3), pg/ml | 42.8 (17.8, 120.4) | 55.8 (19.7, 251.0) | 0.20 | 16.5 (9.7, 37.1) | 18.0 (9.6, 56.4) | 0.39 |
| IL-18, median (Q1, Q3), pg/ml | 575.5 (309.7, 1220.5) | 589.8 (297.1, 1383.7) | 0.85 | 564.9 (296.8, 1449.5) | 582.1 (310.6, 1708.7) | 0.51 |
| TNFR-1, median (Q1, Q3), pg/ml | 1707.6 (1165.6, 2210.4) | 1745.2 (1122.4, 2507.3) | 0.76 | 1558.6 (951.6, 2493.6) | 2019.4 (1315.7, 3086.1) | 0.03 |
| TNFR-2, median (Q1, Q3), pg/ml | 21,180.0 (14,411.3, 26,980.0) | 19,118.4 (12,878.4, 30,340.0) | 0.81 | 20,220.0 (12,126.8, 31,420.0) | 25,290.0 (17,809.1, 40,475.0) | 0.03 |
d0, Blood samples were drawn at the time of randomization; d3, blood samples were drawn 72 h after randomization.
Table 7.
Analysis of pro- and anti-inflammatory markers (RRT at 1 year)
| Patients without RRT at 1 yr, d0 | Patients with RRT at 1 yr, d0 | P Value | Patients without RRT at 1 yr, d3 | Patients with RRT at 1 yr, d3 | P Value | |
|---|---|---|---|---|---|---|
| MIF, median (Q1, Q3), pg/ml | 16,488.6 (10,100.7, 42,386.4) | 12,100.8 (8103.0, 56,378.1) | 0.68 | 10,882.8 (4828.2, 15,916.2) | 6588.0 (2620.2, 35,932.2) | 0.79 |
| IL-6, median (Q1, Q3), pg/ml | 957.3 (295.3, 1697.0) | 2175.0 (1044.4, 2339.1) | 0.12 | 257.6 (100.9, 595.6) | 696.5 (82.0, 764.2) | 0.65 |
| IL-8, median (Q1, Q3), pg/ml | 255.2 (93.7, 553.5) | 334.6 (111.0, 461.8) | 0.94 | 64.2 (28.4, 125.4) | 51.4 (13.6, 97.6) | 0.49 |
| IL-10, median (Q1, Q3), pg/ml | 44.4 (20.8, 129.4) | 26.3 (10.0, 98.6) | 0.27 | 16.5 (8.9, 37.2) | 15.3 (14.2, 33.8) | 0.85 |
| IL-18, median (Q1, Q3), pg/ml | 597.0 (348.6, 1220.5) | 288.8 (194.6, 1179.2) | 0.11 | 539.1 (290.5, 1455.0) | 747.5 (395.9, 1201.8) | 0.69 |
| TNFR-1, median (Q1, Q3), pg/ml | 1707.6 (1205.7, 2202.4) | 1505.0 (866.0, 2926.2) | 0.80 | 1550.7 (943.1, 2411.1) | 2125.0 (990.2, 3217.7) | 0.50 |
| TNFR-2, median (Q1, Q3), pg/ml | 21,180.0 (14,844.9, 26,980.0) | 19,477.1 (9258.5, 28,190.0) | 0.51 | 22,080.0 (12,481.9, 31,440.0) | 18,222.2 (10,872.8, 30,900.0) | 0.51 |
Patients who died within 1 year are excluded; n=8 patients with RRT at 1 year. d0, Blood samples were drawn at the time of randomization; d3, blood samples were drawn 72 h after randomization.
Table 8.
Analysis of pro- and anti-inflammatory markers (PRD; defined as 25% decline in eGFR)
| Pro- and Anti-Inflammatory Markers | Patients without PRD, d0 | Patients with PRD, d0 | P Value | Patients without PRD, d3 | Patients with PRD, d3 | P Value |
|---|---|---|---|---|---|---|
| MIF, median (Q1, Q3), pg/ml | 15,019.2 (10,189.2, 44,648.4) | 16,675.2 (8085.6, 36,628.8) | 0.92 | 11,283.6 (5988.6, 23,681.1) | 6588.0 (3412.8, 12,800.4) | 0.04 |
| IL-6, median (Q1, Q3), pg/ml | 1151.4 (354.1, 1794.9) | 943.9 (210.8, 2175.2) | 0.56 | 282.7 (102.1, 552.1) | 253.6 (100.2, 699.0) | 0.91 |
| IL-8, median (Q1, Q3), pg/ml | 313.6 (123.5, 571.2) | 220.5 (88.6, 443.5) | 0.22 | 73.3 (30.9, 129.2) | 51.4 (16.0, 100.5) | 0.19 |
| IL-10, median (Q1, Q3), pg/ml | 44.6 (20.8, 153.0) | 39.8 (14.9, 101.3) | 0.34 | 22.2 (9.6, 48.6) | 15.3 (9.2, 28.1) | 0.47 |
| IL-18, median (Q1, Q3), pg/ml | 727.0 (343.7, 1153.7) | 565.5 (267.8, 1374.8) | 0.46 | 777.3 (350.9, 1849.6) | 381.2 (241.9, 747.5) | <0.01 |
| TNFR-1, median (Q1, Q3), pg/ml | 1600.3 (1221.1, 2104.9) | 1838.6 (1035.0, 2521.0) | 0.52 | 1447.8 (951.1, 2021.1) | 2058.4 (945.4, 2898.8) | 0.21 |
| TNFR-2, median (Q1, Q3), pg/ml | 22,630.0 (14,873.8, 27,055.0) | 18,577.6 (11,624.6, 27,280.0) | 0.18 | 20,880.0 (11,916.3, 29,420.0) | 20,200.0 (13,090.0, 39,715.0) | 0.48 |
Patients who died within 1 year are excluded; n=39 patients with PRD at 1 year. d0, Blood samples were drawn at the time of randomization; d3, blood samples were drawn 72 h after randomization.
Discussion
In this novel follow-up analysis of the ELAIN Trial, we found that early initiation of RRT significantly reduced the rate of MAKE365. We also found that increased plasma TNFR-1 and TNFR-2 concentrations 72 hours after initiating RRT were associated with an increased MAKE365 rate and 1-year mortality.
In a study by De Corte et al.,21 the rate of major adverse kidney events (MAKEs) in critically ill patients with AKI receiving RRT was 87.5%. This result agrees very well with our finding in the delayed RRT group (89.1%). However, early initiation of RRT was associated with a significantly reduced MAKE rate. In both our study and the study by De Corte et al.,21 MAKE was mainly determined by mortality. Among 1-year survivors, approximately 57% had complete recovery of kidney function. With a dialysis dependence rate of approximately 9.0% in survivors at 1 year, our findings were lower than those reported by De Corte et al.21 (19% at 1 year) and in the AKI Trials Network Study (about 13% at 1 year)22 but higher than in the Randomized Evaluation of Normal Versus Augmented Level Replacement Therapy Study (about 4% at 1 year).23
Our study highlights the increased rate of MAKEs at 1 year associated with AKI treated with RRT in critically ill patients. A little less than one third of randomized patients were alive 1 year after randomization, a lower survival rate than seen in other life-threatening conditions, such as acute respiratory distress syndrome.24 In addition, >40% of the survivors had persistent renal function and/or dialysis dependence. Follow-up of patients in other studies21–23 also revealed that a large proportion of AKI RRT survivors had incomplete renal recovery. These data support the observation that survivors of AKI are at increased risk and that these patients should be referred to a nephrologist so that closer surveillance and optimal therapy can be delivered.
Our findings show the benefits of using MAKE as a composite end point in AKI studies. The components of this composite end point are clearly defined, and MAKE is a clinically important end point. The use of this composite end point includes a greater percentage of patients with poor outcomes, increases the event rate for the assessment of therapies, and prevents the constraint of competing risks associated with single outcomes. However, MAKE as an end point of AKI studies is not without limitations. Because individual components might be affected differently by interventions, when using MAKE as the primary outcome, it is necessary to present all of the individual components.25–27 In this study, MAKE was determined mainly by mortality. Therefore, it is not surprising that variables associated with mortality were also associated with MAKE: timing of RRT and age at randomization. Our results indicate that early initiation of RRT could not establish a benefit in patients >80 years old. However, our cohort of patients >80 years old (n=25) is too small to come to a definitive conclusion.
Several studies showed that elevated concentrations of proinflammatory and apoptosis biomarkers in patients with AKI are associated with an increased short-term mortality.28–31 Increased IL-6 and IL-8 levels early after disease onset predict an increased short-term mortality.28,29 However, only a few studies have investigated whether higher concentrations of biomarkers are associated with long-term outcome.32 Our findings show that, overall, higher levels of MIF, IL-6, IL-8, IL-10, and IL-18 are predictors of 1-year all-cause mortality when adding to clinical factors. Elevated IL-6 concentrations have already been shown to be associated with an increased risk of long-term all-cause mortality in patients after cardiac surgery.33 Strikingly, we found higher IL-10 to be associated with an increased risk for mortality at 1 year, although protective effects have been proposed.33,34 However, in contrast to the TRIBE-AKI Trial, we did not only include patients after cardiac surgery, and the etiology of AKI may play a crucial role. In this study, we showed that the TNFR-1 and TNFR-2 concentrations were significantly higher on day 3 after disease onset in patients who met the MAKE365 end point compared with patients who were MAKE365 negative. One explanation of why IL-6 and -8 levels were not elevated in patients who were MAKE365 positive is that the levels rapidly decrease in the first days after disease onset,32 and we measured these biomarkers 3 days after initiation of RRT. However, these data show an association of elevated TNFR-1 and TNFR-2 concentrations with adverse renal outcome and death in patients with AKI. Engagement of the Fas/TNFR receptor family triggers cell apoptosis. Higher concentrations of TNFR have been associated with susceptibility to AKI and mortality35 as well as failure to recover renal function after AKI.29 Of course, the association between reduced concentrations of mediators and the better outcome does not establish causation. One explanation might be that the early initiation of RRT corrects electrolyte and volume imbalances more rapidly, leading to a reduced inflammatory response and organ damage.
Strengths of these results are the scale and completeness of long-term follow-up. The design avoids potential bias from retrospective selection of the study cohort, allows a better estimation of absolute risk, and enhances the clinical applicability of the results. However, a limitation of such a study design is that the results of such a trial are not always generalizable to other patient populations. Thus, care should be applied in extrapolating these results from critically ill patients to other patients with AKI. Although the follow-up data on mortality and renal outcomes are complete, only a limited selection of biochemical variables is available (eGFR), not necessarily reflecting the actual kidney function.
In a large cohort of critically ill patients with AKI randomized to different initiation strategies of RRT, the increased risk of MAKE continues well beyond 90 days after randomization but can be altered by early initiation of RRT. The proportion of patients with persistent renal dysfunction is high among survivors, suggesting significant ongoing risk of the development of CKD and mortality. The importance of elevated concentrations of TNFR-1 and TNFR-2 as markers of MAKE and mortality is also notable.
Concise Methods
The ELAIN Trial has been described in detail elsewhere.20 Briefly, 231 adult patients were enrolled from August of 2013 to July of 2015. Inclusion criteria were (1) KDIGO stage 2 (twofold increase in serum creatinine from baseline [for baseline serum creatinine, we used the serum creatinine at hospital admission, the last available serum creatinine within the last 3 months, or an estimated serum creatinine as per the KDIGO guideline36 in patients with no information about their prior kidney function] or urinary output <0.5 ml/kg per hour for ≥12 hours) despite optimal resuscitation (optimizing intravascular volume [fluid resuscitation: pulmonary artery occlusion pressure/central venous pressure of >12 mm Hg or stroke volume variation <12% in ventilated patients]; optimization of cardiac index [>2.6 L/min per square meter]; hemodynamic optimization [mean arterial pressure >65 mm Hg]; or normalizing intra-abdominal pressure [<15 mm Hg]); (2) plasma neutrophil gelatinase–associated lipocalin >150 ng/ml; (3) at least one of the following conditions: severe sepsis, use of vasopressors or catecholamines (norepinephrine or epinephrine >0.1 μg/kg per minute), refractory fluid overload (worsening pulmonary edema, PaO2/FiO2<300 mm Hg, or fluid balance >10% of body weight), or development or progression of nonrenal organ dysfunction (SOFA score ≥2); (4) age between 18 and 90 years old; and (5) intention to provide full intensive care treatment for at least 3 days. Patients with preexisting CKD (eGFR<30 ml/min), previous RRT, AKI caused by permanent occlusion or surgical lesion of the renal artery, GN, interstitial nephritis, vasculitis, postrenal obstruction, hemolytic uremic syndrome, or thrombotic thrombocytopenic purpura were excluded. We also excluded patients for pregnancy, prior kidney transplantation, hepatorenal syndrome, AIDS with a CD4 count of <0.05×10 E/L, hematologic malignancy with neutrophils of <0.05×10 E/L, or participation in another interventional clinical trial. Patients were randomized if all inclusion criteria were fulfilled and no exclusion criteria were met on a 1:1 basis stratified by hemodynamic SOFA score (0–2 versus 3–4) and the presence or absence of oliguria to one of the two treatment arms. Early RRT was initiated within 8 hours after diagnosis of KDIGO stage 2. Delayed RRT was initiated within 12 hours after achieving KDIGO stage 3 (greater than threefold increase in serum creatinine or serum creatinine ≥4 mg/dl with an acute increase of at least 0.5 mg/dl within 48 hours or urine output <0.3 ml/kg per hour for ≥24 hours) or if an absolute indication for initiation of RRT was developed (serum urea >100 mg/dl, serum potassium >6 mEq/L and/or with electrocardiography abnormalities, serum magnesium >8 mEq/L, urine production <200 ml/12 hours or anuria, and organ edema in the presence of AKI resistant to diuretic treatment). All patients in both groups were primarily treated with continuous venovenous hemodiafiltration to ensure hemodynamic stability. RRT was discontinued if renal recovery defined by urine output >400 ml/24 hours without or >2100 ml/24 hours with diuretic treatment and creatinine clearance >20 ml/min occurred. If cessation criteria were not fulfilled after 7 days, continuous RRT could be changed to an intermittent technique (sustained low-efficiency daily dialysis, slow continuous ultrafiltration, or intermittent hemodialysis). Further intensive care treatment was performed according to the clinic internal standards. The ELAIN Trial was approved by the Institutional Review Board. All subjects or legally authorized representatives provided written informed consent. The trial is registered at http://www.drks.de (Identifier: DRKS00004367).
Sample and Data Collection
Blood samples were collected by standard methods on the day of randomization and 3 days after randomization. The samples were centrifuged and stored immediately after collection at −80°C until assayed. All clinical data, including patient demographics, serum creatinine, RRT dependency, and mortality, were collected and stored in a password-protected dataset. Telephone calls to the subject, the family members, or the general practitioner were performed for the follow-up information.
Biomarker Assay Methods
All inflammatory mediators (IL-6, IL-8, IL-10, IL-18, TNFR-1, TNFR-2, and MIF) were analyzed according to the manufacturer’s specification using commercially available assay kits LEGENDplex (BioLegend, San Diego, CA).
End Points
The primary end point of this follow-up analysis was the composite end point MAKE365 consisting of death, need for RRT, and persistent renal dysfunction at 1 year after randomization. Persistent renal dysfunction was defined as 25% decline in eGFR compared with baseline. eGFR was calculated with the Cockroft–Gault formula via serum creatinine levels from patients’ medical records (serum creatinine values were determined within 4 weeks before or after the 1-year follow-up). For patients discharged alive, telephone calls to the general practitioner, the subject, or the family members were performed. If the patient met the RRT end point at 1 year, the subject was also defined as persistent renal dysfunction; however, both RRT and death were evaluated separately. MAKE has been recommended by experts, because death is a competing end point.
Our secondary end point was the performance of inflammatory biomarkers for prediction of the primary end point (MAKE365) and its components (mortality, RRT dependence, and persistent renal dysfunction).
Statistical Analyses
In descriptive statistical analyses, non-normally distributed continuous variables were described by median values, Q1, and Q3. Categorical variables were described by absolute and relative frequencies. Overall survival was estimated using the Kaplan–Meier method. Differences between groups were reported as absolute difference, OR, or HR where applicable with corresponding 95% CIs. The primary end point MAKE365 was compared between the early and delayed groups using the chi-squared test with a two-sided significance level α=0.05. The result provides confirmatory statistical evidence. Group differences of secondary and other end points were evaluated using the Mann–Whitney U test, the chi-squared test, and the log rank test. In sensitivity analyses of overall survival, a Cox model with piecewise constant hazard functions with a cut point at 90-days follow-up was fitted. P values of secondary and other end points were regarded ‘significant’ in case P≤0.05. Results were considered exploratory, not confirmatory. Multivariable regression analysis was performed using logistic regression and the Cox Proportional Hazards Model. On the basis of a full model with clinical potentially relevant covariates (sex; age; SOFA score; normoliguria/oliguria; CKD; severe sepsis; fluid balance; Acute Physiology, Age, Chronic Health Evaluation and SOFA score; ASA; heart insufficiency; diabetes; COPD; source of admission; pro- and anti-inflammatory mediators; and early versus delayed RRT initiation), the final model was established by applying backward variable elimination. Statistical analyses were performed using IBM SPSS Statistics for Windows, version 24 (IBM Corp., Armonk, NY) and SAS, version 9.4 (SAS Institute Inc., Cary, NC).
Disclosures
M.M. reports receiving lecture fees from Astute Medical. J.A.K. reports receiving grant support and consulting fees from Astute Medical, Alere, and Baxter. A.Z. reports receiving grant support from Astute Medical, Fresenius, and the German Research Foundation and lecture fees from Astute Medical, Fresenius, Astellas, and Braun. No other disclosures were reported.
Supplementary Material
Acknowledgments
The study was funded by Else-Kröner Fresenius Stiftung grant 2013_A46 (to A.Z.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2017060694/-/DCSupplemental.
References
- 1.Hoste EA, Bagshaw SM, Bellomo R, Cely CM, Colman R, Cruz DN, Edipidis K, Forni LG, Gomersall CD, Govil D, Honoré PM, Joannes-Boyau O, Joannidis M, Korhonen AM, Lavrentieva A, Mehta RL, Palevsky P, Roessler E, Ronco C, Uchino S, Vazquez JA, Vidal Andrade E, Webb S, Kellum JA: Epidemiology of acute kidney injury in critically ill patients: The multinational AKI-EPI study. Intensive Care Med 41: 1411–1423, 2015 [DOI] [PubMed] [Google Scholar]
- 2.Uchino S: The epidemiology of acute renal failure in the world. Curr Opin Crit Care 12: 538–543, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Star RA: Treatment of acute renal failure. Kidney Int 54: 1817–1831, 1998 [DOI] [PubMed] [Google Scholar]
- 4.Liaño F, Junco E, Pascual J, Madero R, Verde E; The Madrid Acute Renal Failure Study Group : The spectrum of acute renal failure in the intensive care unit compared with that seen in other settings. Kidney Int Suppl 66: S16–S24, 1998 [PubMed] [Google Scholar]
- 5.Chertow GM, Christiansen CL, Cleary PD, Munro C, Lazarus JM: Prognostic stratification in critically ill patients with acute renal failure requiring dialysis. Arch Intern Med 155: 1505–1511, 1995 [PubMed] [Google Scholar]
- 6.Wald R, Quinn RR, Luo J, Li P, Scales DC, Mamdani MM, Ray JG; University of Toronto Acute Kidney Injury Research Group : Chronic dialysis and death among survivors of acute kidney injury requiring dialysis. JAMA 302: 1179–1185, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Lo LJ, Go AS, Chertow GM, McCulloch CE, Fan D, Ordoñez JD, Hsu CY: Dialysis-requiring acute renal failure increases the risk of progressive chronic kidney disease. Kidney Int 76: 893–899, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi AI, Li Y, Parikh C, Volberding PA, Shlipak MG: Long-term clinical consequences of acute kidney injury in the HIV-infected. Kidney Int 78: 478–485, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Newsome BB, Warnock DG, McClellan WM, Herzog CA, Kiefe CI, Eggers PW, Allison JJ: Long-term risk of mortality and end-stage renal disease among the elderly after small increases in serum creatinine level during hospitalization for acute myocardial infarction. Arch Intern Med 168: 609–616, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Ishani A, Xue JL, Himmelfarb J, Eggers PW, Kimmel PL, Molitoris BA, Collins AJ: Acute kidney injury increases risk of ESRD among elderly. J Am Soc Nephrol 20: 223–228, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, Tolwani A, Ronco C; Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators : Acute renal failure in critically ill patients: A multinational, multicenter study. JAMA 294: 813–818, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Chertow GM, Levy EM, Hammermeister KE, Grover F, Daley J: Independent association between acute renal failure and mortality following cardiac surgery. Am J Med 104: 343–348, 1998 [DOI] [PubMed] [Google Scholar]
- 13.Chawla LS, Kimmel PL: Acute kidney injury and chronic kidney disease: An integrated clinical syndrome. Kidney Int 82: 516–524, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Coca SG, Singanamala S, Parikh CR: Chronic kidney disease after acute kidney injury: A systematic review and meta-analysis. Kidney Int 81: 442–448, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu PC, Wu CJ, Lin CJ, Wu VC; National Taiwan University Study Group on Acute Renal Failure Group : Long-term risk of upper gastrointestinal hemorrhage after advanced AKI. Clin J Am Soc Nephrol 10: 353–362, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu VC, Wu PC, Wu CH, Huang TM, Chang CH, Tsai PR, Ko WJ, Chen L, Wang CY, Chu TS, Wu KD; National Taiwan University Study Group on Acute Renal Failure (NSARF) Group : The impact of acute kidney injury on the long-term risk of stroke. J Am Heart Assoc 3: e000933, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chawla LS, Amdur RL, Shaw AD, Faselis C, Palant CE, Kimmel PL: Association between AKI and long-term renal and cardiovascular outcomes in United States veterans. Clin J Am Soc Nephrol 9: 448–456, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu VC, Wu CH, Huang TM, Wang CY, Lai CF, Shiao CC, Chang CH, Lin SL, Chen YY, Chen YM, Chu TS, Chiang WC, Wu KD, Tsai PR, Chen L, Ko WJ; NSARF Group : Long-term risk of coronary events after AKI. J Am Soc Nephrol 25: 595–605, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaudry S, Hajage D, Schortgen F, Martin-Lefevre L, Pons B, Boulet E, Boyer A, Chevrel G, Lerolle N, Carpentier D, de Prost N, Lautrette A, Bretagnol A, Mayaux J, Nseir S, Megarbane B, Thirion M, Forel JM, Maizel J, Yonis H, Markowicz P, Thiery G, Tubach F, Ricard JD, Dreyfuss D; AKIKI Study Group : Initiation strategies for renal-replacement therapy in the intensive care unit. N Engl J Med 375: 122–133, 2016 [DOI] [PubMed] [Google Scholar]
- 20.Zarbock A, Kellum JA, Schmidt C, Van Aken H, Wempe C, Pavenstädt H, Boanta A, Gerß J, Meersch M: Effect of early vs delayed initiation of renal replacement therapy on mortality in critically ill patients with acute kidney injury: The ELAIN randomized clinical trial. JAMA 315: 2190–2199, 2016 [DOI] [PubMed] [Google Scholar]
- 21.De Corte W, Dhondt A, Vanholder R, De Waele J, Decruyenaere J, Sergoyne V, Vanhalst J, Claus S, Hoste EA: Long-term outcome in ICU patients with acute kidney injury treated with renal replacement therapy: A prospective cohort study. Crit Care 20: 256, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pike F, Murugan R, Keener C, Palevsky PM, Vijayan A, Unruh M, Finkel K, Wen X, Kellum JA; Biological Markers for Recovery of Kidney (BioMaRK) Study Investigators : Biomarker enhanced risk prediction for adverse outcomes in critically ill patients receiving RRT. Clin J Am Soc Nephrol 10: 1332–1339, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gallagher M, Cass A, Bellomo R, Finfer S, Gattas D, Lee J, Lo S, McGuinness S, Myburgh J, Parke R, Rajbhandari D; POST-RENAL Study Investigators and the ANZICS Clinical Trials Group : Long-term survival and dialysis dependency following acute kidney injury in intensive care: Extended follow-up of a randomized controlled trial. PLoS Med 11: e1001601, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Angus DC, Clermont G, Linde-Zwirble WT, Musthafa AA, Dremsizov TT, Lidicker J, Lave JR; NO-06 Investigators : Healthcare costs and long-term outcomes after acute respiratory distress syndrome: A phase III trial of inhaled nitric oxide. Crit Care Med 34: 2883–2890, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Billings FT 4th, Shaw AD: Clinical trial endpoints in acute kidney injury. Nephron Clin Pract 127: 89–93, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kellum JA, Zarbock A, Nadim MK: What endpoints should be used for clinical studies in acute kidney injury? Intensive Care Med 43: 901–903, 2017 [DOI] [PubMed] [Google Scholar]
- 27.Kellum JA: How can we define recovery after acute kidney injury? Considerations from epidemiology and clinical trial design. Nephron Clin Pract 127: 81–88, 2014 [DOI] [PubMed] [Google Scholar]
- 28.Zarbock A, Gerß J, Van Aken H, Boanta A, Kellum JA, Meersch M: Early versus late initiation of renal replacement therapy in critically ill patients with acute kidney injury (The ELAIN-Trial): Study protocol for a randomized controlled trial. Trials 17: 148, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murugan R, Wen X, Shah N, Lee M, Kong L, Pike F, Keener C, Unruh M, Finkel K, Vijayan A, Palevsky PM, Paganini E, Carter M, Elder M, Kellum JA; Biological Markers for Recovery of Kidney (BioMaRK) Study Investigators : Plasma inflammatory and apoptosis markers are associated with dialysis dependence and death among critically ill patients receiving renal replacement therapy. Nephrol Dial Transplant 29: 1854–1864, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simmons EM, Himmelfarb J, Sezer MT, Chertow GM, Mehta RL, Paganini EP, Soroko S, Freedman S, Becker K, Spratt D, Shyr Y, Ikizler TA; PICARD Study Group : Plasma cytokine levels predict mortality in patients with acute renal failure. Kidney Int 65: 1357–1365, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Liu KD, Glidden DV, Eisner MD, Parsons PE, Ware LB, Wheeler A, Korpak A, Thompson BT, Chertow GM, Matthay MA; National Heart, Lung, and Blood Institute ARDS Network Clinical Trials Group : Predictive and pathogenetic value of plasma biomarkers for acute kidney injury in patients with acute lung injury. Crit Care Med 35: 2755–2761, 2007 [PMC free article] [PubMed] [Google Scholar]
- 32.Kellum JA, Kong L, Fink MP, Weissfeld LA, Yealy DM, Pinsky MR, Fine J, Krichevsky A, Delude RL, Angus DC; GenIMS Investigators : Understanding the inflammatory cytokine response in pneumonia and sepsis: Results of the Genetic and Inflammatory Markers of Sepsis (GenIMS) study. Arch Intern Med 167: 1655–1663, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang WR, Garg AX, Coca SG, Devereaux PJ, Eikelboom J, Kavsak P, McArthur E, Thiessen-Philbrook H, Shortt C, Shlipak M, Whitlock R, Parikh CR; TRIBE-AKI Consortium : Plasma IL-6 and IL-10 concentrations predict AKI and long-term mortality in adults after cardiac surgery. J Am Soc Nephrol 26: 3123–3132, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deng J, Kohda Y, Chiao H, Wang Y, Hu X, Hewitt SM, Miyaji T, McLeroy P, Nibhanupudy B, Li S, Star RA: Interleukin-10 inhibits ischemic and cisplatin-induced acute renal injury. Kidney Int 60: 2118–2128, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Iglesias J, Marik PE, Levine JS; Norasept II Study Investigators : Elevated serum levels of the type I and type II receptors for tumor necrosis factor-alpha as predictive factors for ARF in patients with septic shock. Am J Kidney Dis 41: 62–75, 2003 [DOI] [PubMed] [Google Scholar]
- 36.KDIGO AKI Work Group : KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2: 1–138, 2012 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.