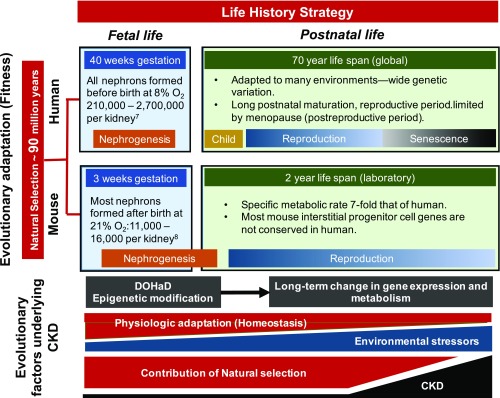
Figure 1.
Evolutionary factors play a central role in determining life history strategy, which spans fetal and postnatal life, and underlies differences between human and mouse kidney development as well as the course of CKD. Evolutionary divergence of mouse and human is estimated at 85-94 million years ago, the result of evolutionary adaptation driven by reproductive fitness (vertical axis). Differences in nephrogenesis between species are the product of natural selection over that interval, and reflect evolutionary adaptation to life history strategies spanning fetal and postnatal periods (horizontal axis). Human nephrogenesis is complete before the end of gestation, taking place in an 8% oxygen environment. Murine nephrogenesis begins in fetal life, but most nephrons are formed after birth in 21% ambient oxygen. In contrast to the mouse, the number of nephrons in the human kidney at birth is highly variable and subject to epigenetic modification of nephrogenesis in response to fetal environmental stimuli (developmental programming). A new discipline, the Developmental Origins of Health and Disease (DOHaD), is defining the role of this process in long-term gene expression, with reduced nephron number at birth now recognized as a risk factor for CKD in adulthood. Adapted to many environments with wide genetic variation, human life history strategy includes prolonged childhood followed by a reproductive and postreproductive period (menopause). In contrast, specific metabolic activity in the mouse is seven-fold that of human, a reflection of its small size (greater surface area/volume ratio) and greater metabolic demands. Mesenchymal progenitor cell types and transcriptional profiles are also markedly divergent in mouse and human nephrogenesis. In contrast to evolutionary adaptation, physiologic adaptation (such as nephron hypertrophy following reduced nephron number) is a short-term response that is less robust with advancing age, during which time accumulating environmental stressors also contribute to increasing CKD. Because natural selection is driven by reproductive fitness, its contribution to human evolution is largely limited in late adulthood (senescence), during which nephron number normally decreases by 50%, accounting for a sharp rise in CKD during this period.