Figure 1. Enrollment and Inclusion in Analysis in the Nulliparous Pregnancy Outcome Study: Monitoring Mothers-to-Be (nuMoM2b).
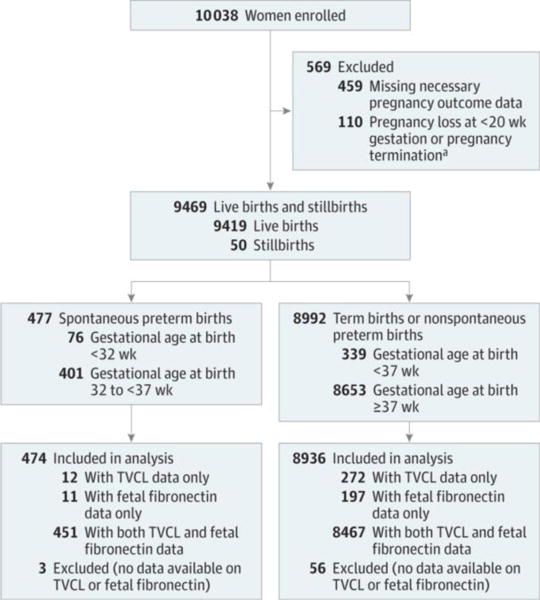
This analysis assessed serial transvaginal ultrasound cervical length and quantitative vaginal fetal fibronectin, alone and in combination, and measured at different points during pregnancy and as rates of change, to detect spontaneous preterm birth in nulliparous women with singleton pregnancies successfully carried 20 weeks or more. Women enrolled in the study who carried their pregnancy to 20 weeks or more were eligible for the analysis. To determine eligibility required collection of the necessary pregnancy outcome data to exclude pregnancy losses at less than 20 weeks. Furthermore, the analysis was only possible among the women with results from at least 1 serial transvaginal ultrasound or 1 sample assayed for fetal fibronectin level. TVCL indicates transvaginal cervical length.
aElective termination (n = 10), indicated termination (n = 23), and fetal demise at less than 20 weeks’ gestational age (n = 77).
