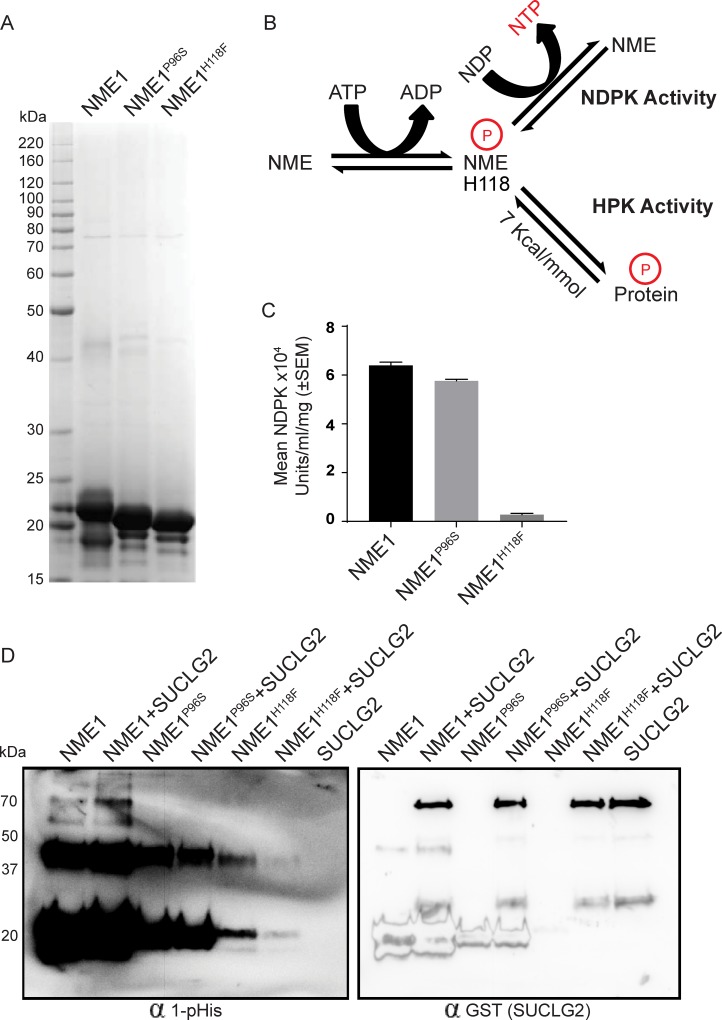
Figure 8. NME1P96S recombinant protein provides a correlation of HPK activity with motility suppression.
A. Recombinant NME1, NME1P96S and NME1H118F constructs were His tagged, expressed in E. coli and partially purified by affinity purification using immobilized metal affinity chromatography. A coomassie stained 10-20% gradient SDS-PAGE gel of the partially purified proteins is shown. B. Diagrammatic representation of NME activities; NME1 is reversibly auto-histidine phosphorylated (H118) in presence of ATP. This phosphate, via a high energy phosphohistidine bond on NME1, is transferred reversibly to either a nucleotide diphosphate in NDPK activity or to a protein in HPK activity. C. The proteins were assessed for their NDPK activity by a spectrophotometric assay. D. The proteins were assessed for their HPK activity using SUCLG2 as their substrate. NME1, NME1P96S or NME1H118F (400 ng) were incubated with SUCLG2 (600 ng) in TMD buffer containing ATP for 30’ at room temperature. Reaction was stopped by adding 5x lysis buffer and 1-phosphohistidine western protocol was used for the detection of 1-pHis; after stripping, SUCLG2 (GST tagged) was detected by anti-GST antibody. Histidine phosphorylated SUCLG2 appears in NME1+SUCLG2 lane at ~70kDa. 1-pHis band at ~20 kDa and ~37 kDa are histidine phosphorylated monomer and dimer of NME1.