Fig. 1.
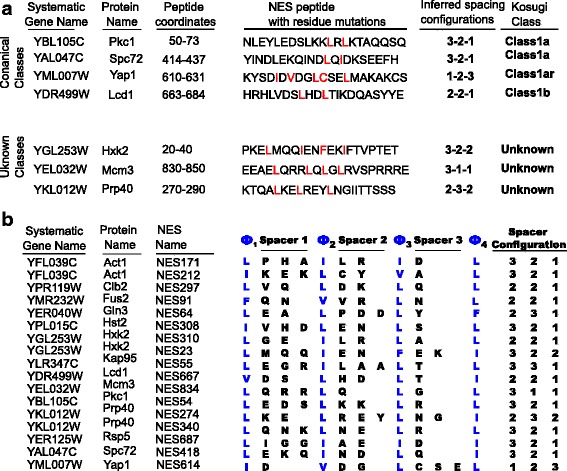
Analysis of high-confidence S. cerevisiae NESs. a shows unaligned 21 amino acid peptide window of 4 yeast NESs with canonical spacing configurations (upper panel) and 3 yeast NESs with spacing patterns that are inconsistent with any of the canonical spacing configurations (lower panel). The residues demonstrated through mutagenesis studies to be important for NES function are highlighted in red. (See Additional file 1: Table S1 for details) b NESs manually aligned with gaps based on their hydrophobic positions. The numbers in the NES names refer to amino acid position of first hydrophobic residue
