Table 5.
Results for studies reporting excess arm volume (ml)
| Study | Method | Number of participants (n) | Pre-intervention EAV | Post-interventionEAV | Final reportedEAV |
| Complex decongestive treatment (CDT/DLT) | |||||
| Dayes et al. 201335,aDLT groupb,cControl groupb,c | Manual circumference measurement | 3123 | 23% ± 1221% ± 7 | 17% ± 12e12% ± 12e | 15% ± 15e15% ± 13e |
| Gradalski et al. 201582CDT groupb,dCB group | Manual circumference measurement | 25‡ | 898 ± 445‡ | 472 ± 285e,f‡ | 506 ± 263e‡ |
| Hwang et al. 201379CDT group 1 (<20%EAV)b,dCDT group 2 (≥20%EAV) | Perometer | 32‡ | 11% ± 5‡ | 10% ± 9g‡ | 14% ± 11g‡ |
EAV = excess arm volume.
‡Data not presented: mean breast cancer-related lymphedema (BCRL) duration >9 months.
aData provided by corresponding author.
bData presented as relative (%) EAV, i.e. 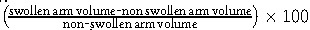 .
.
cSubgroup of women with BCRL duration <12months.
dMean BCRL duration ≤9 months.
ep > .05 between group difference.
fp < .05 within group difference.
gNo p values reported.
