Fig. 1.
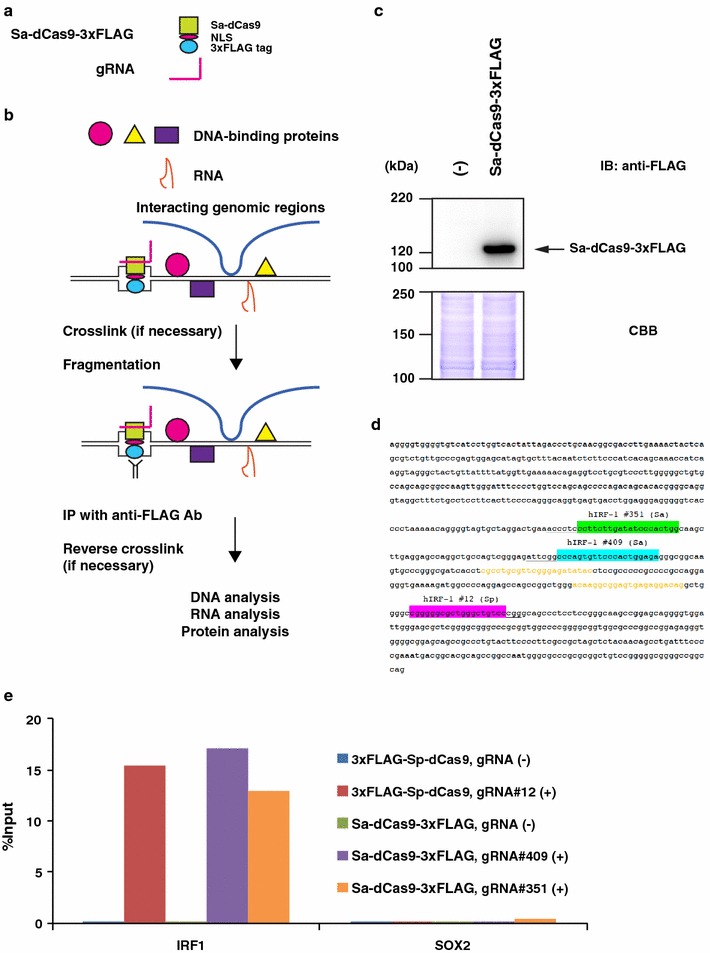
enChIP system using S. aureus CRISPR. a The S. aureus CRISPR system for enChIP. The system is composed of a fusion protein, Sa-dCas9-3xFLAG (consisting of Sa-dCas9, an NLS, and a 3xFLAG-tag) and a gRNA. b Scheme of the enChIP system using S. aureus CRISPR. The Sa-dCas9-3xFLAG and gRNA are expressed for locus-tagging in the target cells. The cells are crosslinked (if necessary), lysed, and fragmented by sonication or other methods. Chromatin complexes containing the CRISPR complex are immunoprecipitated with anti-FLAG Ab, and the crosslink (if used) is reversed. Molecules (DNA, RNA, proteins, etc.) associated with the target genomic region can be identified by downstream analyses (e.g., nucleic acids by next-generation sequencing, proteins by mass spectrometry). c Expression of Sa-dCas9-3xFLAG. Plasmid expressing Sa-dCas9-3xFLAG was transfected into 293T cells. Nuclear extracts were prepared and subjected to immunoblot analysis (IB) with anti-FLAG Ab. Coomassie Brilliant Blue (CBB) staining is shown as a protein loading control. d Positions of gRNAs in the IRF-1 promoter. Green highlight: hIRF-1 #351 (Sa) gRNA; blue highlight: hIRF-1 #409 (Sa) gRNA; magenta highlight: hIRF-1 #12 (Sp) gRNA; underlines: PAM sequences; orange letters: primers for enChIP-PCR analysis. e Isolation of the IRF-1 locus by enChIP using the S. aureus CRISPR system. Real-time PCR analysis of chromatin complexes isolated by enChIP is shown. An irrelevant locus (SOX2) was analysed as a negative control. The S. pyogenes CRISPR system was used as a positive control for enChIP
