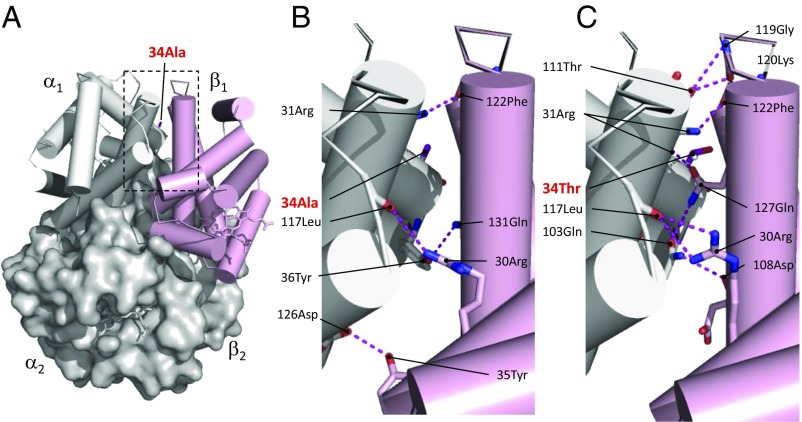
Fig. 3.
The αAA34T substitution in Parus humilis and Lophophanes dichrous increases Hb–O2 affinity by adding new hydrogen bonds that stabilize the oxygenated R conformation of the Hb tetramer relative to the deoxygenated T conformation. (A) Structural model of avian Hb in the liganded R state. (B) A zoomed-in view of the intradimer α1β1 interface of R-state Hb with the ancestral Ala at αA34. Four hydrogen bonds (depicted as dashed lines) are predicted at this interface. (C) Replacing Ala with Thr at α34 produces a twofold increase in the number of hydrogen bonds at the interface (Table S3), thereby increasing the stability of the R state.