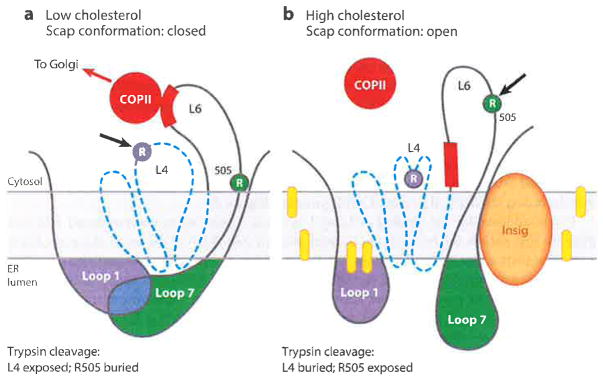
Figure 8.
Model for cholesterol-mediated conformational change in Scap. (a) When in the low-cholesterol state, Loop 1 is bound to Loop 7, creating a closed conformation. The trypsin cleavage site in Loop 4 (L4) is accessible (arrow), whereas the cleavage site at R505 in Loop 6 (L6) is blocked. (b) When in the high-cholesterol state, the trypsin cleavage site in Loop 4 (L4) is not accessible, Insig is bound to the SSD, and Loop 7 is dissociated from Loop 1, creating an open conformation. R505 of Loop 6 (L6) is accessible to trypsin (arrow), and the MELADL (red bar) is not accessible to COPII proteins. Dashed blue line denotes SSD (transmembrane helices 2–6). Yellow ovals denote cholesterol molecules. Abbreviations: COPII, coat protein II; ER, endoplasmic reticulum; MELADL sequence, methionine-glutamate-leucine-alanine-aspartate-leucine; SSD, sterol-sensing domain. Adapted from Reference 63.