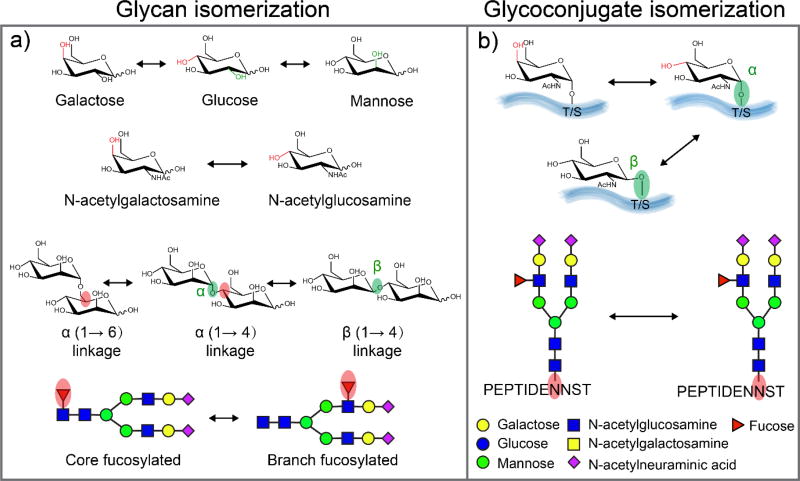
Figure 1.
The isomerization of glycan and glycoconjugates. a) The building blocks (monosaccharides) that compose larger glycans are structural isomers (Hexose: galactose, glucose, mannose, N-acetylhexosamine: N-acetylgalactosamine, N-acetylglucosamine); monosaccharides can be connected either α- or β-stereochemistry at multiple potential linkage position; fucose could be either attached to N-glycan core or branches. b) Epimeric glycoconjugates results from alternative configurations (α- or β-) at the anomeric linkages or the presence of epimeric glycan monomers (galactose or glucose), scheme modified from reference [43]; two isomeric N-glycopeptides differ in the site of N-glycan attachment.