Figure 1. Mechanics of Data Sharing via the ClinicalStudyDataRequest.com (CSDR) Platform.
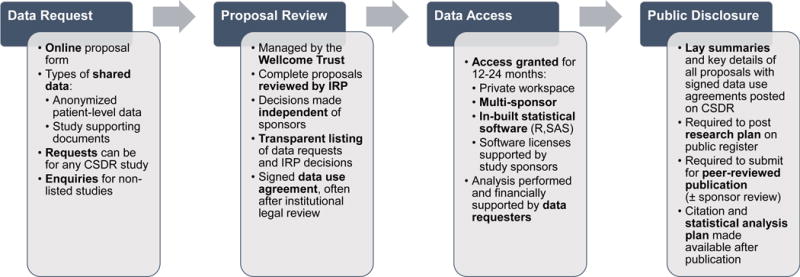
This flow diagram highlights the 4 major steps of this data sharing model including the initial request for access to clinical trial data, review by an Independent Review Panel (IRP), access to a multi-sponsor analysis system, and public dissemination of secondary research findings.
