Figure 2. Availability and Metrics of Use of Cardiometabolic Clinical Trial Data Hosted on the Multi-Sponsor Data Sharing Platform, ClinicalStudyDataRequest.com.
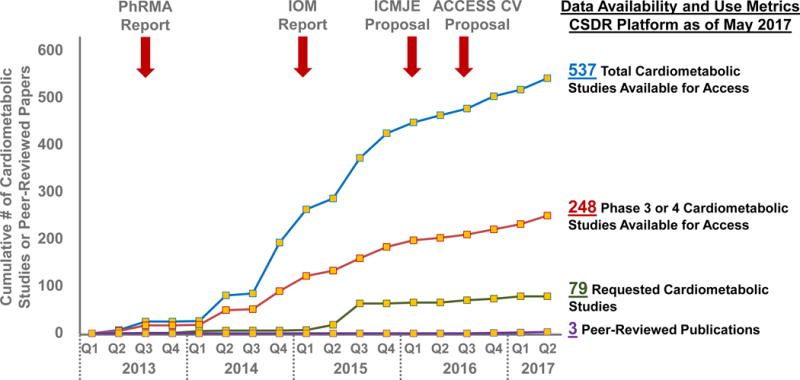
Landmark working group reports and proposals endorsing access to individual patient-level data have been highlighted (red arrows). The blue and red lines reflect availability of data from completed cardiometabolic clinical trials. The green line reflects dates that each cardiometabolic study was first requested through this platform with signed data sharing agreements. The purple line reflects peer-reviewed publications based on these shared data identified through PubMED/MEDLINE. Abbreviations: ACCESS CV = Academic Research Organization Consortium for Continuing Evaluation of Scientific Studies – Cardiovascular; CSDR = ClinicalStudyDataRequest.com; ICMJE = International Committee of Medical Journal Editors; IOM = Institute of Medicine; PhRMA = Pharmaceutical Research and Manufacturers of America.
