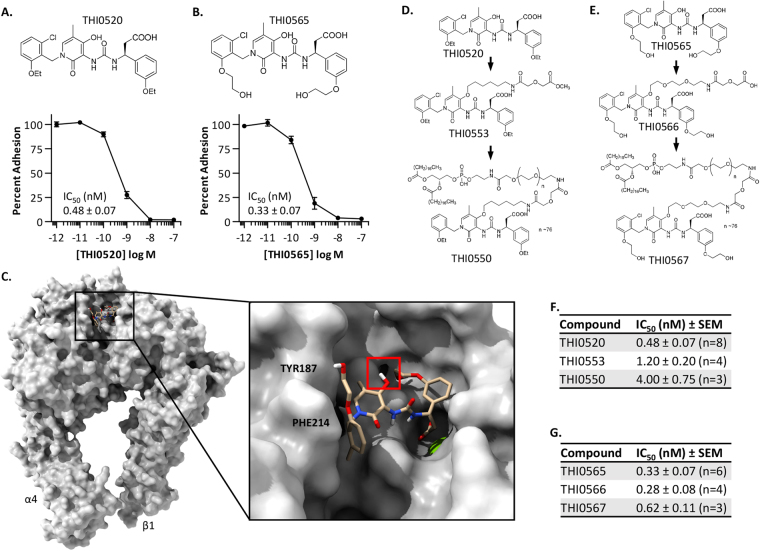
Figure 1.
Modification of small molecule antagonists of the integrin α4β1. (A,B) Structure and activity of the small molecule integrin antagonists. Studies showing α4β1-expressing-K562 cell adhesion to plastic-immobilized vascular cell adhesion molecule-1 (VCAM-1). An average “Percent Adhesion” from at least 6 independent experiments is shown (average ± SEM). (C) Molecular Dynamics simulation of THI0565 binding into the integrin α4β1 ectodomain. THI0565 is anchored by the carboxylic acid coordination of the β1 MIDAS Mg++ ion (green sphere, expanded 2x for visualization) and the 2-hydroxyethoxyphenyl group hydrophobic interactions with α4 residues PHE214 and TYR187. The pyridone hydroxyl appears readily available for modification (red square). (D,E) Modifications of THI0520 and THI0565 to generate targeting conjugates for liposome formulation. (F,G) Inhibitory activity (IC50) of modified compounds as determined in α4β1-K562 cell adhesion assays to VCAM-1.