Fig. 2.
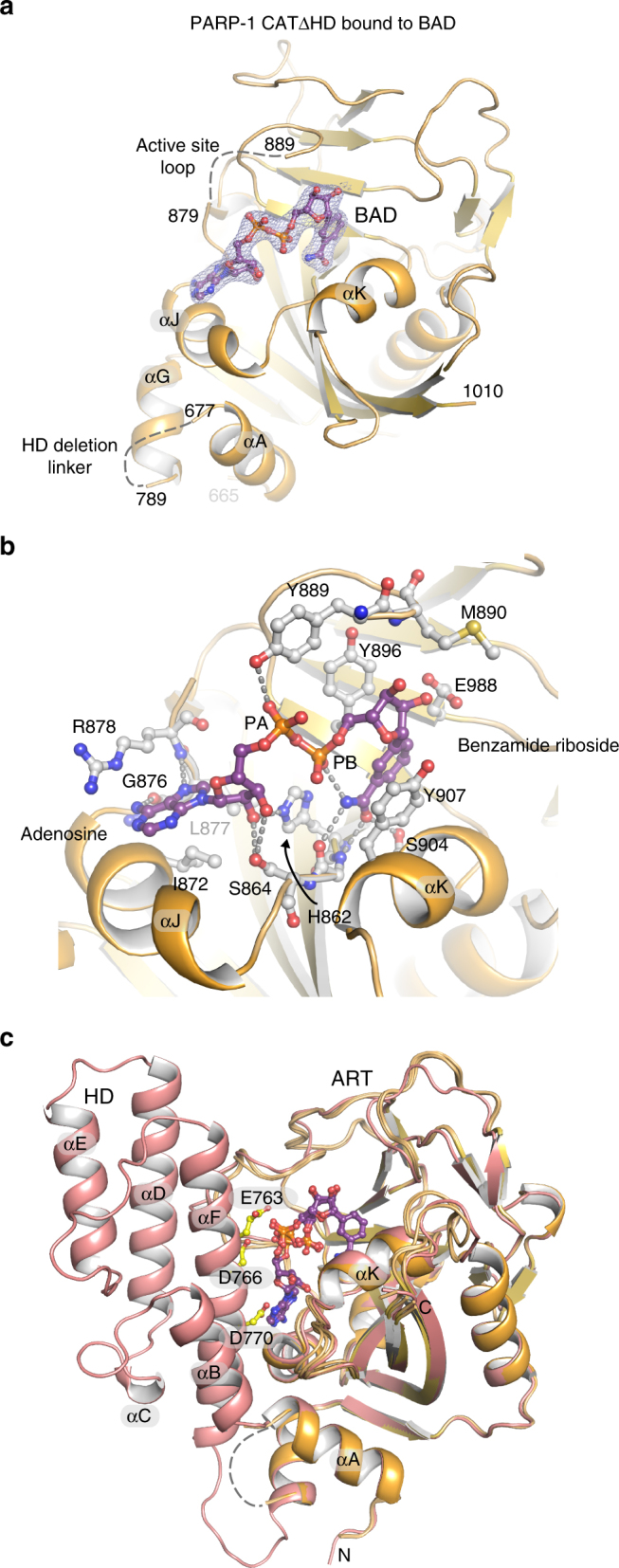
Crystal structure of PARP-1 CAT ΔHD bound to NAD+ analog BAD. a Crystal structure of the PARP-1 CAT domain carrying a HD deletion (residues 661–1011 with an 8-residue linker GSGSGSGG replacing residues 678–787) bound to BAD at 2.3 Å resolution. Four PARP-1 CAT ΔHD molecules were present in the crystal asymmetric unit; molecule C is shown. A 2.3 Å 2FO−FC weighted electron density contoured at 1.2 σ in the region of the bound BAD molecule is overlaid on the structure (see also Supplementary Fig. 1a, b). b Close-up view of the NAD+ binding site and contacts formed with the NAD+ analog BAD in molecule C. c The crystal structure of human PARP-1 CAT domain WT (pdb: 3gjw)40 aligned with the PARP-1 CAT ΔHD/BAD structure
