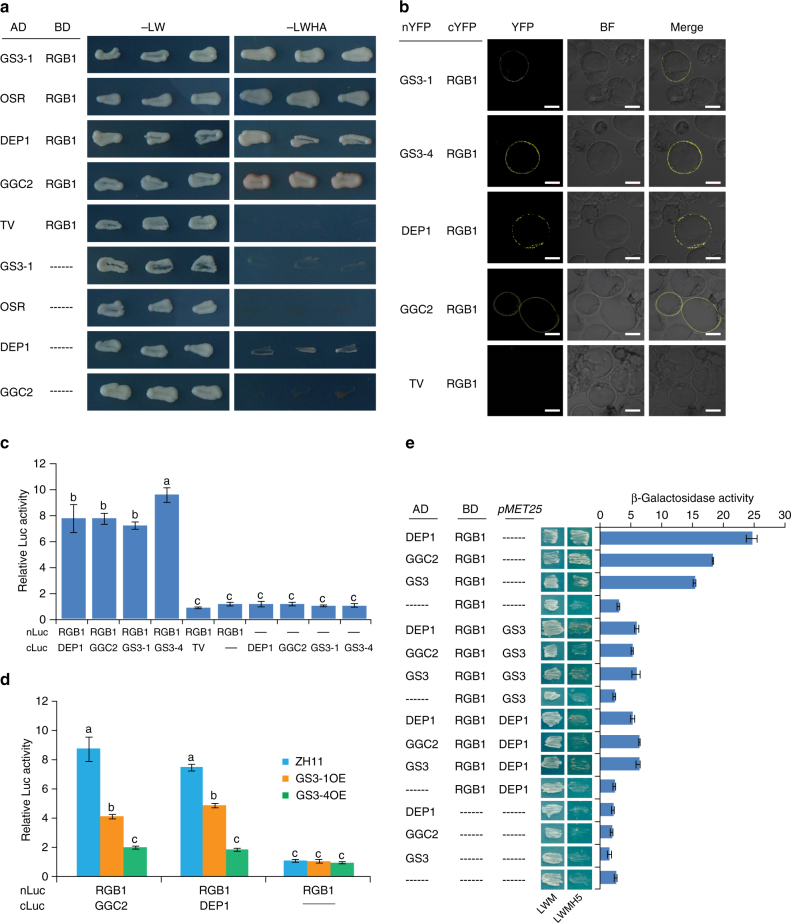
Fig. 5.
Competition of the Gγ proteins in interacting with RGB1. a Interactions of GS3, DEP1 and GGC2 with RGB1 using yeast two-hybrid assay. GS3 interacts with RGB1 through the OSR domain but not the C-terminal cysteine-rich domain (TV). OSR: OSR domain of GS3, amino acid 1-94; TV: cysteine-rich domain of GS3, amino acid 95-231. AD: GAL4 activation domain; BD: GAL4 binding domain. -LW: selective medium lacking Trp and Leu; -LWHA: selective medium lacking Trp, Leu, His and Ade. b Interactions of GS3, DEP1 and GGC2 with RGB1 using BiFC assay. Bar = 20 μm. c Interactions of GS3, GGC2 and DEP1 (fused to C-terminal fragment of firefly luciferase) with RGB1 (fused to N-terminal fragment of firefly luciferase) using luciferase activity assay. Rice protoplasts with transient expression of RGB1-nLuc plus cLuc-DEP1, cLuc-GGC2, cLuc-GS3-1, and cLuc-GS3-4, but not the C-terminal cysteine-rich domain (TV), show high luciferase activity relative to the control. Empty vectors of cLuc plus RGB1-nLuc, and nLuc plus cLuc-DEP1, cLuc-GGC2, cLuc-GS3-1, and cLuc-GS3-4 were used as the negative control. Data are normalized to the internal control 35 S::REN. Values are given as mean ± SEM (n = 3). Different letters indicate significant differences ranked by the LSD test (P < 0.05). d Interactions of DEP1 and GGC2 with RGB1 in the background of ZH11, GS3-1OE, and GS3-4OE using luciferase activity assay. RGB1-nLuc plus cLuc-DEP1, and RGB1-nLuc plus cLuc-GGC2 are transiently expressed in the protoplasts of ZH11, GS3-1OE, and GS3-4OE, respectively. Empty vector of cLuc plus RGB1-nLuc is used as the negative control. Data are normalized to the internal control 35 S::REN. Values are given as mean ± SEM (n = 3). Different letters indicate significant differences ranked by the LSD test (P < 0.05). e Yeast three-hybrid assay for protein interactions of DEP1/GGC2 and GS3 with RGB1. The interaction of DEP1/GGC2 and GS3 with RGB1 is analyzed using fusions with AD (AD-DEP1, GGC2 or GS3) and BD (BD-RGB1). Empty vectors are used as a negative control. Quantitative analysis of interactions by β-galactosidase assay is shown. Data for all the assays are shown as mean ± SD (n = 3)