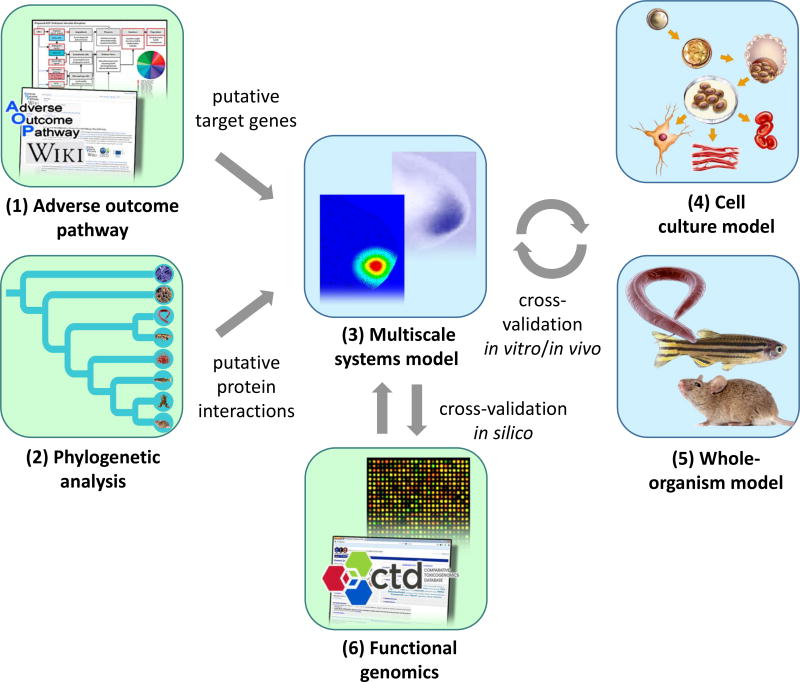
Figure 4. Evolutionary approach to cross-species extrapolation.
Adverse Outcome Pathway (AOP) is a useful framework to integrate a large volume of toxicity data from research literature and high-throughput screening for predicting developmental toxicity in humans. An adverse outcome can be linked to a molecular initiating event (MIE) that involves a developmental toxicant and a molecular target. Risk assessment can be started with the bioinformatics analysis (green) of (1) an AOP to predict MIEs and target genes and (2) phylogenetic analysis of putative protein interactions. Multiscale systems models (3) can be built to examine the spatio-temporal dynamics of developmental patterning. The results of computer simulations can be cross-validated in vitro, in vivo, and in silico using (4) cell culture models, (5) whole-organism models, and (6) functional genomics. This approach can improve the design and data interpretation with different experimental models (blue), thereby increasing the capability in identifying potential developmental toxicants in humans.