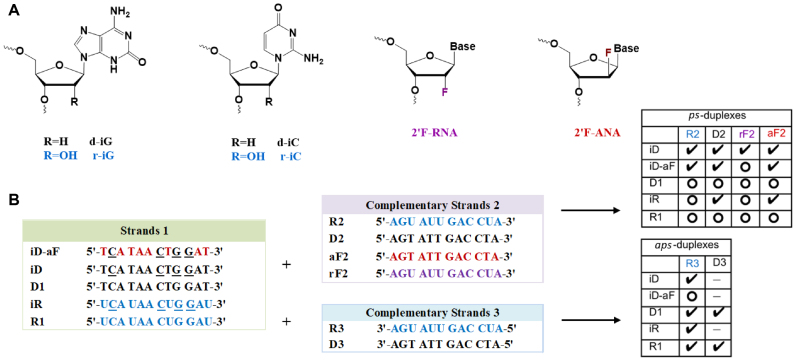
Figure 2.
(A) Structures of chemically modified nucleotides incorporated in ps- and aps-duplexes. (B) Sequence of the synthesized dodecamers for parallel and antiparallel strand pairings. Underlined residues (N) contain the isonucleobase modifications. Check marks indicate duplexes with significant melting transitions; open circles refer to combinations that did not yield a melting transition (see Table 1). “-" means “not measured".