Abstract
The antibody response to RNA-related antigens such as Sm/RNP requires the endosomal RNA sensor TLR7, and this process is crucial in the development of systemic lupus erythematosus at least in animal models. The inhibitory B cell receptor CD72 is unique because it recognizes Sm/RNP and specifically inhibits the activation of Sm/RNP-reactive B cells by activating SH2-containing protein tyrosine phosphatase 1 (SHP-1). In the normal immune system, Sm/RNP-reactive B cells are tolerized by a unique mechanism that probably involves SHP-1. These self-reactive B cells appear in the peripheral lymphoid organs, differentiate into marginal zone B cells, and then undergo apoptosis without further maturation into plasma cells. Thus, CD72 is involved in the suppression of TLR7-mediated response to RNA in complexes with nuclear proteins that are resistant to nucleases, whereas free RNAs are degraded by nucleases before they encounter the endosomal RNA sensor.
Keywords: CD72, Sm/RNP, marginal zone B cell, TLR7, SLE, self-reactive B cell
Introduction
Innate immune cells such as macrophages and dendritic cells (DCs) express receptors recognizing molecules that are broadly expressed by pathogens, including nucleic acids (NAs), known as pathogen-associated molecular patterns (PAMPs).1) These receptors are called pattern recognition receptors (PRRs), and they play a crucial role in triggering acquired immune responses as well as innate responses. Recognition of PAMPs by PRRs in innate immune cells is essential for the induction of acquired immune responses. The recognition of PAMPs activates DCs, major antigen-presenting cells, and makes them capable of presenting antigens to T lymphocyte (T cells), the event required for T cell activation (Fig. 1A). Alternatively, upon recognition of PAMPs, innate immune cells such as macrophages produce inflammatory cytokines, including tumor necrosis factor (TNF) α, which in turn activate DCs. Antigen presentation by DCs specifically activates T cells reactive to the antigen, and activated T cells then interact with B lymphocytes (B cells) that are stimulated by the same antigen. Through this T cell-B cell interaction, B cells are stimulated by T cell-derived signals such as CD40L (CD154) essential for the survival and activation of antigen-stimulated B cells, followed by differentiation to antibody-producing cells.2,3) Thus, the activation of DCs by direct recognition of PAMPs by PRRs or indirectly through cytokines secreted by PAMPs-activated innate cells plays a crucial role in the activation of both T lymphocytes and B lymphocytes and production of specific antibodies to pathogens.
Figure 1.
Distinct involvement of PRRs in antibody response to Sm/RNP. (A) Antibody responses to conventional microbial antigens. Microbe-derived PAMPs activate DCs directly through PRRs. Alternatively, PAMPs stimulate innate cells, including macrophages, to secrete inflammatory cytokines such as TNFα, which activate DCs. Activated DCs acquire antigen presentation activity by increasing the expression of MHC-II and the T cell co-stimulatory molecules CD80 and CD86. Antigenic peptides derived from endocytosed pathogens are presented together with MHC-II, thereby activating T cells specific to microbial antigens. B cells specific to microbial antigens recognize antigens by BCR, and present antigenic peptides together with MHC-II, which interacts with activated T cells with the same antigen specificity. Through this interaction, B cells interact with the T cell-derived co-stimulatory molecule CD40L. The presence of both BCR signaling and CD40 signaling, B cells are activated and produce specific antibodies including those against non-PAMP microbial antigens. PRRs are required for the activation of DCs, and PRRs in B cells play an auxiliary role in antibody production. (B) Antibody responses to NA-related antigens. When B cells reactive to NA-related antigens such as Sm/RNP interact with the antigen through the BCR, the antigens are internalized and then interact with PRRs reactive to NAs (NA sensors) in the endosome. In the presence of BCR signaling and co-stimulatory signaling through NA sensors, B cells are activated and produce antibodies to NA-related antigens. PRRs in B cells are required for antibody responses to NA-related antigens.
Antibodies to NAs and NA-related antigens are characteristically produced in systemic lupus erythematosus (SLE), a prototype systemic autoimmune disease, and play a crucial role in the pathophysiology of SLE. PRR-mediated recognition is essential for the production of autoantibodies to NAs,4–6) as is the case for antibody production in response to conventional antigens including pathogens. However, how PRRs are required in the production of anti-NA antibodies is distinct from that in antibody production in response to conventional antigens. In antibody responses to conventional antigens, PAMPs are involved in the activation of DCs. Once activated, DCs present various antigens, including non-PAMP antigens such as protein components of microbes, thereby triggering antibody production against various microbial antigens (Fig. 1A). Most PRRs are expressed in B cells as well as innate cells, but PRRs expressed in B cells play an auxiliary role in antibody responses to conventional antigens.6,7) In contrast, PRRs in B cells especially those recognizing NAs, are specifically required for the production of anti-NA antibodies (Fig. 1B). B cells recognize antigens via the B cell receptor (BCR) composed of membrane-bound immunoglobulin (Ig) and the signal transducing component Igα (CD79a)/Igβ (CD79b). Recognition of antigens by the BCR induces signaling through the BCR, but BCR signaling alone is not sufficient for B cell activation but induces apoptosis. B cells require a co-stimulatory signal together with antigen recognition for B cell activation. CD40L (CD154) expressed in activated T cells is a predominant costimulatory signal during T cell help to B cells.2,3) However, NA sensors also induce signaling that supports the activation of antigen-stimulated B cells.8,9) When B cells reactive to NA or NA-containing self-antigens recognize these antigens, signaling through BCR together with signaling through NA sensors induces activation of self-reactive B cells, leading to production of anti-NA antibodies. Thus, NA sensors in B cells are specifically required for the production of anti-NA antibodies by co-stimulating B cells that recognize NAs or NA-related antigens.
NA sensors are classified according to their specificity (RNA vs. DNA) and intracellular localization (endosome vs. cytosol).10) Production of anti-NA antibodies in mouse models that spontaneously develop SLE-like autoimmune disease requires NA sensors localized in endosomes such as TLR7 and TLR9.4–6) TLR7 and TLR9 recognize RNA and DNA, respectively, and are required for the production of autoantibodies to RNA-related antigens and DNA, respectively. Of note, mouse lupus models no longer develop diseases in the absence of TLR7, whereas TLR9 deficiency exacerbates the disease due to compensatory enhancement of TLR7 expression.11,12) This result suggested that immune responses to RNA but not DNA are crucial in the development of lupus though it is not yet clear why RNA-related self-antigens but not DNA ones are pathogenic. Patients with SLE produce autoantibodies to RNA-related self-antigens such as ENA and Sm/RNP, among which Sm/RNP, a complex of RNA and nuclear antigens such as U1-SnRNP, is an endogenous ligand of TLR7.9) Thus, immune responses to Sm/RNP appear to be important in development of lupus. This review discusses the molecular and cellular mechanisms that have been elucidated in the regulation of antibody responses to Sm/RNP.
CD72 is an inhibitory receptor that regulates the development of lupus-like disease
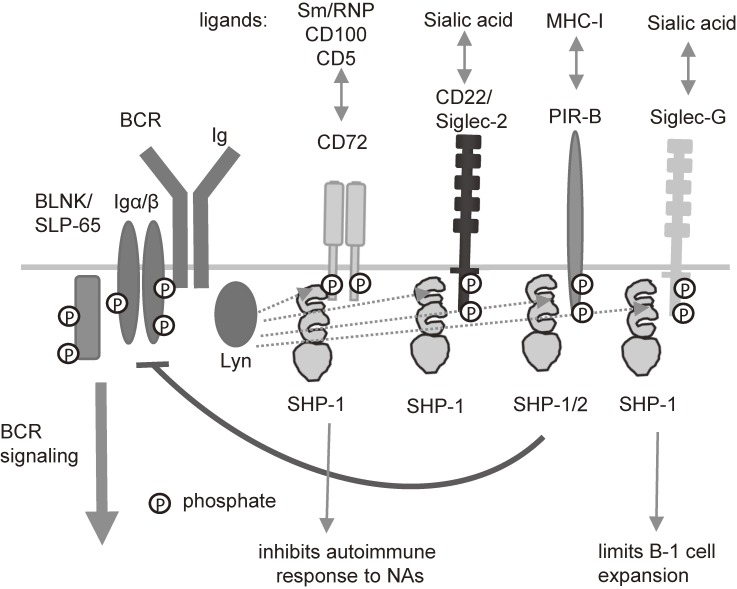
CD72 is a 45-kDa type II membrane protein mostly expressed in B cells. CD72 contains a C-type lectin-like domain (CTLD) in the extracellular part, and was previously reported to bind to CD513) and CD100.14) CD72 also contains an immunoreceptor tyrosine-based inhibition motif (ITIM) in the cytoplasmic region.15,16) When the cytoplasmic ITIM is phosphorylated, CD72 recruits SH2-containing protein tyrosine phosphatase 1 (SHP-1) to the phosphorylated ITIM and activates it. Activated SHP-1 down-modulates BCR signaling by dephosphorylating various BCR signaling molecules including SLP-65/BLNK. B cells express other ITIM-containing membrane molecules such as CD22, Siglec-G/Siglec-10, PIR-B, and PECAM-1.17) These molecules negatively regulate BCR signaling by recruiting SH2-containing phosphatases including SHP-1 to phosphorylated ITIMs. These inhibitory receptors are collectively called B cell inhibitory co-receptors.
There are polymorphisms in both human and mouse CD72. In mouse, three allelic forms, i.e., CD72a, CD72b, and CD72c, are known.18) CD72c contains a number of amino acid substitutions and a 7-amino acid deletion in the extracellular region compared with other allelic forms, whereas CD72a and CD72b are highly homologous. CD72c is carried by lupus prone MRL-Faslpr/lpr mice. The Faslpr/lpr mutation is a loss-of-function mutation of the gene encoding Fas, a member of the TNF receptor family transmitting apoptotic signaling.19) Faslpr/lpr causes severe lupus-like disease in the MRL background, but not on other mouse strains such as C57BL/6.20) Thus, the MRL background contains the genes that induce severe lupus in the presence of Faslpr/lpr. Previously, a genetic study revealed that CD72c is associated with the disease in MRL-Faslpr/lpr mice,21) suggesting that CD72c is a candidate of the MRL genes that cause lupus-like disease in the presence of Faslpr/lpr.
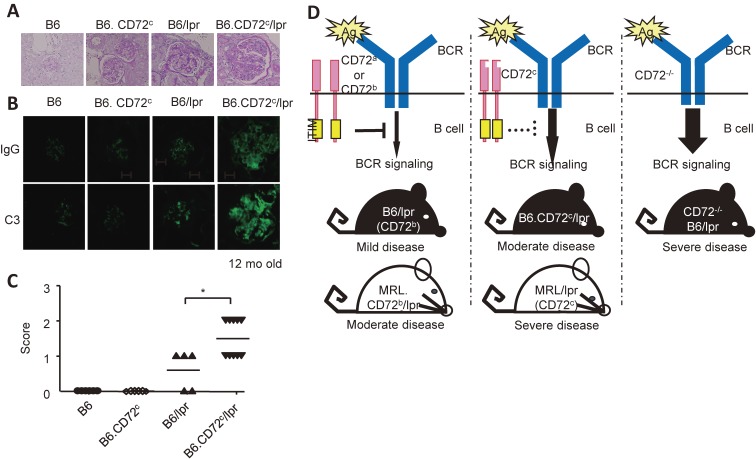
To address the role of CD72c in development of lupus, we generated C57BL/6 mice congenic for CD72c (C57BL/6.CD72c). Whereas C57BL/6.CD72c mice did not develop disease, C57BL/6-Faslpr/lpr. CD72c mice developed moderate lupus-like disease22) (Fig. 2A). Thus, CD72c does not induce autoimmune disease by itself, but induces the disease in the presence of Faslpr/lpr. Conversely, the disease activity is reduced in MRL-Faslpr/lpr mice congenic for CD72b compared with MRL-Faslpr/lpr that carries CD72c. We also demonstrated that CD72−/− C57BL/6 and CD72−/− C57BL/6.Faslpr/lpr mice develop moderate and severe lupus-like disease, respectively. All these data indicated that CD72 inhibits development of lupus, and that CD72c is a functionally weak allele probably due to altered interaction with the ligand.
Figure 2.
CD72 regulates the development of lupus-like disease in mice. (A-C) CD72c, a weak allele, causes moderate lupus-like disease in the C57BL/6 (B6) background in the presence of the Faslpr mutation. Representative PAS staining (A) and immunohistochemistry for IgG and C3 (B) of kidney sections, and scores of the severity of glomerulonephritis are shown. Originally published in The Journal of Immunology (Xu, M. et al., 2013). CD72c is a modifier gene that regulates Faslpr-induced autoimmune disease (J. Immunol. 190: 3189–3196, 2013, Copyright© The American Association of Immunologists, Inc.). (D) Severity of the lupus-like disease in C57BL/6 (B6)-Faslpr/lpr (lpr) and MRL-Faslpr/lpr (lpr) mice shows an inverse correlation with the functional activity of CD72. The MRL background contains additional SLE-causing gene(s) other than CD72c, because mice with the MRL background show more severe disease than mice with the C57BL/6 background with the same CD72 allele.
There are polymorphisms in human CD72, and these polymorphisms have been shown to be associated with SLE using a candidate gene analysis,23) although association of CD72 with SLE has not yet been demonstrated by a genome-wide association study, probably because there are no known polymorphisms that considerably alter the functional activity of CD72.
CD72 specifically regulates B cell responses to Sm/RNP
Although CD72 regulates the development of lupus, CD72 regulates BCR signaling only weakly when BCR is polyclonally ligated using an anti-IgM antibody.22) In contrast, other inhibitory co-receptors such as CD22 and PIR-B strongly regulate BCR signaling induced by an anti-IgM antibody but only weakly regulate development of lupus.24–26) Indeed, mice deficient in CD22 or PIR-B do not develop autoimmune diseases, and develop a mild disease when combined with deficiency in other genes including Faslpr/lpr.
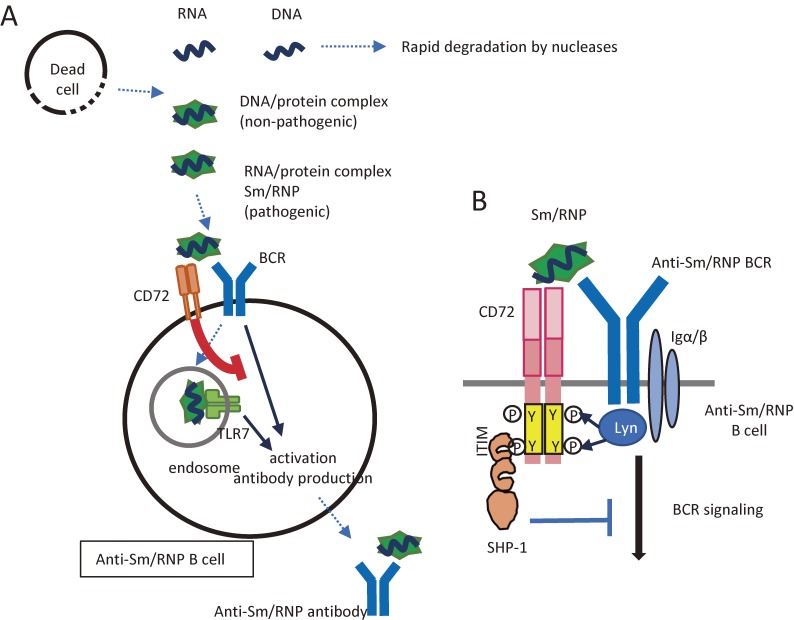
Our recent findings on CD72-mediated signal regulation explain why CD72 strongly regulates the development of lupus without regulating anti-IgM-induced BCR signaling. Previously, the inhibitory activity of CD72 was shown to be down-modulated by interaction with CD100.14) However, activating ligands of CD72 were not known. We recently demonstrated that the CTLD of CD72 recognizes Sm/RNP, an RNA-related self-antigen crucial in the development of lupus, as mentioned above, but not other self-antigens including DNA. This recognition induces CD72-mediated signal inhibition in B cells that produce an anti-Sm/RNP antibody.27) As a result, CD72 inhibits B cell responses to Sm/RNP but not a control antigen (Fig. 3A). The detailed mechanism is as follows. When BCR interacts with Sm/RNP, Sm/RNP co-ligates BCR and CD72, thereby bringing CD72 into close proximity with BCR. This enables BCR-activated kinases such as Lyn to phosphorylate CD72 ITIM, leading to the recruitment of SHP-1 to CD72 (Fig. 3B). Indeed, CD72 is specifically phosphorylated and associated with SHP-1 when BCR interacts with Sm/RNP but not when BCR is ligated by a control antigen. Because CD72 inhibits BCR ligation only when BCR is ligated by Sm/RNP, polyclonal BCR signaling induced by anti-IgM does not appear to be regulated by CD72. In contrast, specific inhibition of B cell responses to Sm/RNP mediated by CD72 may efficiently prevent the development of lupus because the immune response to Sm/RNP is essential for development of this disease.
Figure 3.
CD72 induces self-tolerance to NAs. (A) CD72 maintains self-tolerance to NAs. Among self-NAs, free NAs are rapidly degraded by nucleases after release from dead cells before they reach endosomes. In contrast, NAs complexed with proteins are resistant to nucleases and are able to stimulate endosomal NAs. Antibody responses to the complexes of DNA and proteins are non-pathogenic. The complexes of RNA and proteins such as Sm/RNP are recognized by CD72. This recognition inhibits activation of B cells reactive to the self-RNA/protein complexes and inhibits the production of pathogenic autoantibodies to these self-antigens. (B) Mechanisms for antigen-specific inhibition of B cells by CD72. When B cells that express Sm/RNP-reactive BCR interact with Sm/RNP, CD72 is recruited to BCR through binding to Sm/RNP. ITIM in CD72 is then tyrosine-phosphorylated by BCR-associated kinases, such as Lyn, and recruits and activates SHP-1, which in turn inactivates BCR signaling by dephosphorylating various signaling molecules. In B cells reactive to other antigens, CD72 is not recruited to BCR, and is thus unable to regulate BCR signaling.
As already mentioned, CD72c is a functionally weak allele and is involved in the development of severe lupus-like disease in MRL-Faslpr/lpr mice. SPR analysis using recombinant CD72 CTLD protein revealed that the binding affinity of CD72c CTLD to Sm/RNP was weaker than that of CD72a CTLD.27) Weaker binding to Sm/RNP may make CD72c suppress B cell responses to Sm/RNP only weakly, leading to susceptibility to lupus-like disease. X ray crystallography analysis of CD72a CTLD demonstrated that the surface charge distribution at the putative ligand binding site of CD72c is considerably different from that of CD72a. Probably because of the distinct charge distribution at the putative ligand binding site, CD72c binds to Sm/RNP with a compromised affinity.
Role of CD72 in self-non-self discrimination of NA-related antigens
NAs are crucial PAMPs, but they are also self-antigens crucial in the development of lupus. NA sensors discriminate microbial NAs from self-NAs based on structure and localization.10) For instance, RIG-I located in the cytosol prefers blunt end 5′-triphosphate dsRNA. In the cytosol, blunt end 5′-triphosphate dsRNA is exclusively derived from bacteria or viruses and not the host. Nonetheless, NA sensors recognize self-NAs as well as microbial NAs, and recognition of self-NAs contributes to autoimmunity.
Degradation by nucleases also plays a role in self-non-self recognition of NAs. NAs released by dead cells are rapidly degraded by nucleases in tissue and blood before they are endocytosed and recognized by endosomal PPRs (Fig. 3A), whereas microbial NAs are protected from nucleases by their localization inside microbes. Thus, endosomal localization makes NA-reactive PRRs preferentially respond to microbial NAs but not free NAs released from dead cells. Indeed, when TLR9 is expressed on the cell surface, surface TLR9 induces lupus28) probably by recognizing NAs released from dead cells. However, NAs in complexes with nuclear proteins such as Sm/RNP are resistant to nucleases, and are capable of activating endosomal TLRs. CD72-mediated inhibition of B cell responses to Sm/RNP suppresses immune response to nuclease-resistant self NAs. Thus, CD72 plays a role in self-non-self discrimination of NAs by suppressing immune response to nuclease-resistant self-NAs.
Segregation of B-1 cell expansion and autoimmunity
SHP-1 plays an important role in maintaining tolerance of self-reactive B cells, because conditional B cell-specific deletion of SHP-1 induces activation of self-reactive B cells and production of autoantibodies.29) Moreover, mice deficient in SHP-1 in B cells spontaneously develop lupus-like disease.30) Thus, SHP-1 plays a crucial role in the prevention of lupus-like disease by maintaining self-tolerance of B cells. B-1 cells are distinct from conventional B cells in both development and function. B-1 cells constitute a first line of defense by producing natural cross-reactive antibodies that bind to various pathogens.31) B-1 cells are also suggested to be involved in the development of autoimmune diseases, because they produce cross-reactive autoantibodies and expand in some autoimmune disease models, including mice deficient in SHP-1 in B cells.30,32) In B cells, SHP-1 mediates the inhibitory functions of various inhibitory B cell co-receptors including CD72 (Fig. 4). Because CD72−/− mice develop moderate lupus-like disease,22) CD72 appears to be a major inhibitory co-receptor that activates SHP-1 to prevent autoimmunity. Of note, CD72−/− mice do not show B-1 cell expansion. In contrast, B-1 cells are expanded in mice deficient in Siglec-G,26) another inhibitory B cell co-receptor, although these mice do not develop autoimmune diseases. Thus, development of lupus-like disease is not necessarily associated with B-1 cell expansion. Although both of autoimmunity and B-1 cell expansion are regulated by SHP-1, these two phenotypes are regulated by distinct inhibitory co-receptors, i.e., CD72 and Siglec-G, that activate SHP-1.
Figure 4.
SHP-1 regulates autoimmune responses to NAs and B-1 cell expansion through distinct inhibitory receptors. B cells express various inhibitory receptors containing ITIMs. These receptors share activation and effector mechanisms, including SHP-1 activation. In contrast, these receptors recognize distinct self-antigens. CD72 recognizes Sm/RNP and inhibits autoantibody responses to self NAs. In contrast, Siglec-G recognizes sialic acid, and regulates B-1 cell expansion.
Self-tolerance of B cells reactive to Sm/RNP
It is already well established that, in healthy individuals, self-reactive B cells are either deleted, inactivated (anergy) or converted to non-self-reactive B cells by replacement of the Ig V genes (receptor editing), thereby preventing self-reactive B cells to expand and to produce autoantibodies.33) When self-reactive B cells develop in bone marrow, they are tolerized before migration to the peripheral lymphoid organs such as the spleen and lymph nodes. However, self-tolerance of B cells reactive to Sm/RNP is somewhat distinct from that of other self-reactive B cells.
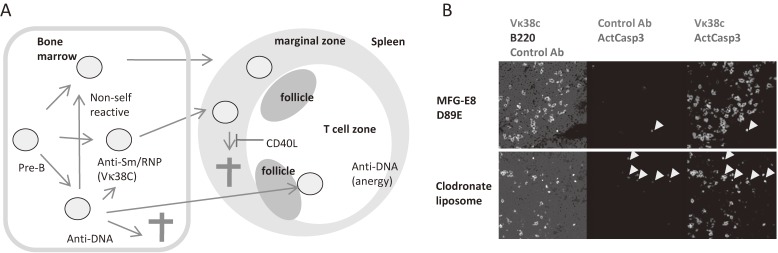
Mice transgenic for the Ig H chain 3H9 and its variants established by Weigert and colleagues are useful in analyzing the self-tolerance of B cells producing anti-nuclear autoantibodies, because these Ig H chains generate anti-nuclear antibodies by associating with various Ig L chains.34–36) When we analyzed the mice transgenic for the 3H9 variant, in which aspartic acid at position 56 was replaced by arginine (56R), we noticed that B cells that reacted with Sm/RNP mostly utilized a single Vκ, i.e., Vκ38C, together with 56R H chain to recognize Sm/RNP. The analysis using monoclonal antibody that specifically recognizes the combination of 56R H chain and Vκ38C revealed that Sm/RNP-reactive B cells are present at a high frequency in transitional B cells, which are peripheral B cells at the stage earlier than mature peripheral B cells, and at a lower frequency in marginal zone (MZ) B cells37) (Fig. 5A). Peripheral lymphoid organs contain two distinct B cell populations, i.e., follicular B cells and MZ B cells. MZ B cells are located in the MZ in the spleen and in the subcapsular sinus in lymph nodes, and play a distinct role such as antibody responses to polysaccharide antigens.38) Because CD40L, a T cell-derived costimulatory signal, is overexpressed in patients with SLE, we generated mice overexpressing CD40L39) and crossed them with 56R-transgenic mice. Excess CD40L expands Sm/RNP-reactive MZ B cells probably by suppressing apoptosis of these B cells, and induces their differentiation into antibody-producing cells.37) Indeed, apoptotic Sm/RNP-reactive B cells are found in splenic MZ in 56R mice when macrophages are depleted by clodronate liposome (Fig. 5B), indicating that Sm/RNP-reactive MZ B cells undergo apoptosis and removed by macrophages. These results indicated that Sm/RNP-reactive B cells migrate to peripheral lymphoid organs and differentiate into MZ B cells, and that Sm/RNP-reactive MZ B cells are deleted by apoptosis, thereby maintaining self-tolerance whereas excess CD40L induces survival of these self-reactive MZ B cells and autoantibody production.
Figure 5.
Self-tolerance of Sm/RNP-reactive B cells. (A) Anti-DNA B cells are either deleted, functionally inactivated (anergy), or converted to non-self-reactive B cells by replacement of Ig V genes. Anergized B cells migrate to the border between the follicle and T cell zone in peripheral lymphoid organs. In contrast, Sm/RNP-reactive B cells appear in the marginal zone of the spleen, and undergo apoptosis, which is blocked by excess CD40L. In 56R-trasngenic mice, Sm/RNP-reactive B cells mostly express Vκ38C. (B) Sm/RNP-reactive B cells undergo apoptosis in the splenic marginal zone and are efficiently removed by macrophages. When macrophage-mediated removal of apoptotic cells is blocked by either treatment with a dominant negative form of MFG-E8 (MFG-E8 D89E) (upper panel) or by removal of macrophages using clodronate liposomes (lower panel) in 56R mice, active caspase 3-containing cells (arrow heads) are observed. Most of these apoptotic cells express Vκ38C. Originally published in ref. 35).
Self-reactive B cells that are anergized in bone marrow migrate to peripheral lymphoid organs.40) Anergized B cells are typically localized at the border between the T cell zone and follicle but not MZ40) in the periphery, and excess CD40L fails to reverse anergy.41) Thus, classical anergy does not appear to be induced in Sm/RNP-reactive B cells. In contrast, CD72 recognizes Sm/RNP and activates SHP-1,27) crucial in maintenance of anergy.29) SHP-1 activated by CD72 in Sm/RNP-reactive B cells may cause a functionally impaired status distinct from classical anergy.
Autoimmune disease in CD72−/− mice is accelerated by Faslpr/lpr.22) Fas is a transmembrane receptor that transmits pro-apoptotic signaling, and is highly expressed in germinal center B cells,42,43) which extensively proliferate in follicles after activation by antigens and T cell help. Because deletion of Fas in class-switched B cells was sufficient for induction of autoimmunity,44) Fas may be involved in apoptotic deletion of self-reactive B cells probably in germinal centers after B cells are activated and undergo class switching. Thus, CD72 and Fas appear to maintain the tolerance of self-reactive B cells in peripheral lymphoid organs at different B cell differentiation stages and by distinct mechanisms, and their deficiency synergistically induce autoimmune disease.
Conclusion
PRRs play a crucial role in activating acquired immunity by inducing antigen-presentation activity of DCs in antibody responses to conventional antigens. Although PRRs are expressed in B cells, PRRs expressed in B cells play an auxiliary role in antibody responses to conventional antigens. In contrast, PRRs especially NA-reactive TLRs expressed in B cells play a crucial role in anti-NA antibody production. This is probably because NA-reactive TLRs transmit co-stimulatory signaling to activate NA-reactive B cells that recognize NAs as antigens, resulting in anti-NA autoantibody production. Although it has not been discussed in this review, PRR-mediated responses of DCs to NAs are also crucial in the development of SLE.45,46) DCs uptake the complex of NA-related antigens and autoantibodies, and respond to NAs via recognition by endosomal TLRs, resulting in the production of type 1 interferon (IFN). Augmented expression of IFN-stimulated genes is the most characteristic feature of the gene expression in leucocytes from SLE patients, and is called the IFN signature.47,48) IFN appears to activate various immune cell types and contributes to the breakdown of B cell self-tolerance. Thus, PRRs play a distinct role in development of SLE, i.e., activation of NA-reactive B cells for autoantibody production, and the production of IFN in DCs.
Among various NA sensors, the endosomal RNA sensor TLR7 plays a central role in the development of SLE, at least in various animal models. Although free RNA released from dead cells is rapidly degraded by nucleases, RNA complexed with nuclear proteins such as Sm/RNP is relatively resistant to nucleases and is able to interact with TLR7 in endosomes after endocytosis. Therefore, RNA–protein complexes such as Sm/RNP appear to be a major TLR7 ligands involved in the development of SLE. By specifically recognizing Sm/RNP, CD72 inhibits B cell signaling by activating SHP-1 in Sm/RNP-reactive B cells. Sm/RNP-reactive B cells are present in peripheral lymphoid tissues, but are tolerized by a distinct mechanism presumably by SHP-1 activation. Thus, CD72 maintains self-tolerance to NAs by inhibiting B cell response to Sm/RNP, which is a nuclease-resistant and pathogenic NA-related self-antigen. However, how CD72-deficiency perturbs self-tolerance and causes lupus-like disease together with Fas-deficiency is not yet fully elucidated. Further studies are needed to elucidate the cellular processes regulated by CD72 and Fas in the regulation of autoimmunity.
Abbreviations
- BCR
B cell receptor
- CTLD
C-type lectin-like domain
- DC
dendritic cell
- IFN
interferon
- Ig
immunoglobulin
- ITIM
immunoreceptor tyrosine-based inhibition motif
- MZ
marginal zone
- NA
nucleic acid
- PAMP
pathogen-associated molecular pattern
- PRR
pattern recognition receptor
- SLE
systemic lupus erythematosus
- TNF
tumor necrosis factor
Profile
Takeshi Tsubata was born in Kyoto in 1957, and graduated from Kyoto University School of Medicine in 1981. He initially majored in internal medicine, and received his Ph.D. from Kyoto University in 1988. He worked at the Institute for Genetics University of Cologne and Max Planck Institute for Immunobiology (Freiburg) in Germany as an Alexander von Humboldt fellow from 1989 to 1991. He then worked at the Department of Medical Chemistry Kyoto University School of Medicine, and was appointed as associate professor in 1993. Since 1996, he has been a full professor at the Department of Immunology, Medical Research Institute, Tokyo Medical and Dental University. He was the Dean, School of Biomedical Sciences, Tokyo Medical and Dental University from 2003 to 2010. He discovered multiple mechanisms for the regulation of immunity versus immunological tolerance of B lymphocytes, which play crucial roles in the regulation of antibody responses and the development of autoimmune diseases. For his achievements in immunology and also scientific exchange between Japan and Germany, he received the Philipp Franz von Siebold Prize from the Federal President of Germany, and the Eugen und Ilse Seibold Prize from the German Research Foundation. He has been an associate member of the Science Council of Japan since 2006.
References
- 1).Kawai T., Akira S. (2009) The roles of TLRs, RLRs and NLRs in pathogen recognition. Int. Immunol. 21, 317–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2).Tsubata T., Wu J., Honjo T. (1993) B-cell apoptosis induced by antigen receptor crosslinking is blocked by a T-cell signal through CD40. Nature 364, 645–648. [DOI] [PubMed] [Google Scholar]
- 3).Garside P., Ingulli E., Merica R.R., Johnson J.G., Noelle R.J., Jenkins M.K. (1998) Visualization of specific B and T lymphocyte interactions in the lymph node. Science 281, 96–99. [DOI] [PubMed] [Google Scholar]
- 4).Christensen S.R., Shupe J., Nickerson K., Kashgarian M., Flavell R.A., Shlomchik M.J. (2006) Toll-like receptor 7 and TLR9 dictate autoantibody specificity and have opposing inflammatory and regulatory roles in a murine model of lupus. Immunity 25, 417–428. [DOI] [PubMed] [Google Scholar]
- 5).Savarese E., Steinberg C., Pawar R.D., Reindl W., Akira S., Anders H.J., et al. (2008) Requirement of Toll-like receptor 7 for pristane-induced production of autoantibodies and development of murine lupus nephritis. Arthritis Rheum. 58, 1107–1115. [DOI] [PubMed] [Google Scholar]
- 6).Soni C., Wong E.B., Domeier P.P., Khan T.N., Satoh T., Akira S., et al. (2014) B cell-intrinsic TLR7 signaling is essential for the development of spontaneous germinal centers. J. Immunol. 193, 4400–4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).Teichmann L.L., Schenten D., Medzhitov R., Kashgarian M., Shlomchik M.J. (2013) Signals via the adaptor MyD88 in B cells and DCs make distinct and synergistic contributions to immune activation and tissue damage in lupus. Immunity 38, 528–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Leadbetter E.A., Rifkin I.R., Hohlbaum A.M., Beaudette B.C., Shlomchik M.J., Marshak-Rothstein A. (2002) Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature 416, 603–607. [DOI] [PubMed] [Google Scholar]
- 9).Lau C.M., Broughton C., Tabor A.S., Akira S., Flavell R.A., Mamula M.J., et al. (2005) RNA-associated autoantigens activate B cells by combined B cell antigen receptor/Toll-like receptor 7 engagement. J. Exp. Med. 202, 1171–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Schlee M., Hartmann G. (2016) Discriminating self from non-self in nucleic acid sensing. Nat. Rev. Immunol. 16, 566–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Santiago-Raber M.L., Dunand-Sauthier I., Wu T., Li Q.Z., Uematsu S., Akira S., et al. (2010) Critical role of TLR7 in the acceleration of systemic lupus erythematosus in TLR9-deficient mice. J. Autoimmun. 34, 339–348. [DOI] [PubMed] [Google Scholar]
- 12).Nickerson K.M., Christensen S.R., Shupe J., Kashgarian M., Kim D., Elkon K., et al. (2010) TLR9 regulates TLR7- and MyD88-dependent autoantibody production and disease in a murine model of lupus. J. Immunol. 184, 1840–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Van de Velde H., von Hoegen I., Luo W., Parnes J.R., Thielemans K. (1991) The B-cell surface protein CD72/Lyb-2 is the ligand for CD5. Nature 351, 662–665. [DOI] [PubMed] [Google Scholar]
- 14).Kumanogoh A., Watanabe C., Lee I., Wang X., Shi W., Araki H., et al. (2000) Identification of CD72 as a lymphocyte receptor for the class IV semaphorin CD100: a novel mechanism for regulating B cell signaling. Immunity 13, 621–631. [DOI] [PubMed] [Google Scholar]
- 15).Adachi T., Flaswinkel H., Yakura H., Reth M., Tsubata T. (1998) The B cell surface protein CD72 recruits the tyrosine phosphatase SHP-1 upon tyrosine phosphorylation. J. Immunol. 160, 4662–4665. [PubMed] [Google Scholar]
- 16).Adachi T., Wakabayashi C., Nakayama T., Yakura H., Tsubata T. (2000) CD72 negatively regulates signaling through the antigen receptor of B cells. J. Immunol. 164, 1223–1229. [DOI] [PubMed] [Google Scholar]
- 17).Tsubata T. (2012) Role of inhibitory BCR co-receptors in immunity. Infect. Disord. Drug Targets 12, 181–190. [DOI] [PubMed] [Google Scholar]
- 18).Robinson W.H., Ying H., Miceli M.C., Parnes J.R. (1992) Extensive polymorphism in the extracellular domain of the mouse B cell differentiation antigen Lyb-2/CD72. J. Immunol. 149, 880–886. [PubMed] [Google Scholar]
- 19).Watanabe-Fukunaga R., Brannan C.I., Copeland N.G., Jenkins N.A., Nagata S. (1992) Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature 356, 314–317. [DOI] [PubMed] [Google Scholar]
- 20).Izui S., Kelley V.E., Masuda K., Yoshida H., Roths J.B., Murphy E.D. (1984) Induction of various autoantibodies by mutant gene lpr in several strains of mice. J. Immunol. 133, 227–233. [PubMed] [Google Scholar]
- 21).Qu W.M., Miyazaki T., Terada M., Lu L.M., Nishihara M., Yamada A., et al. (2000) Genetic dissection of vasculitis in MRL/lpr lupus mice: a novel susceptibility locus involving the CD72c allele. Eur. J. Immunol. 30, 2027–2037. [DOI] [PubMed] [Google Scholar]
- 22).Xu M., Hou R., Sato-Hayashizaki A., Man R., Zhu C., Wakabayashi C., et al. (2013) Cd72(c) is a modifier gene that regulates Fas(lpr)-induced autoimmune disease. J. Immunol. 190, 5436–5445. [DOI] [PubMed] [Google Scholar]
- 23).Hitomi Y., Tsuchiya N., Kawasaki A., Ohashi J., Suzuki T., Kyogoku C., et al. (2004) CD72 polymorphisms associated with alternative splicing modify susceptibility to human systemic lupus erythematosus through epistatic interaction with FCGR2B. Hum. Mol. Genet. 13, 2907–2917. [DOI] [PubMed] [Google Scholar]
- 24).Ujike A., Takeda K., Nakamura A., Ebihara S., Akiyama K., Takai T. (2002) Impaired dendritic cell maturation and increased T(H)2 responses in PIR-B(−/−) mice. Nat. Immunol. 3, 542–548. [DOI] [PubMed] [Google Scholar]
- 25).Kubo T., Uchida Y., Watanabe Y., Abe M., Nakamura A., Ono M., et al. (2009) Augmented TLR9-induced Btk activation in PIR-B-deficient B-1 cells provokes excessive autoantibody production and autoimmunity. J. Exp. Med. 206, 1971–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26).Jellusova J., Wellmann U., Amann K., Winkler T.H., Nitschke L. (2010) CD22 x Siglec-G double-deficient mice have massively increased B1 cell numbers and develop systemic autoimmunity. J. Immunol. 184, 3618–3627. [DOI] [PubMed] [Google Scholar]
- 27).Akatsu C., Shinagawa K., Numoto N., Liu Z., Ucar A.K., Aslam M., et al. (2016) CD72 negatively regulates B lymphocyte responses to the lupus-related endogenous toll-like receptor 7 ligand Sm/RNP. J. Exp. Med. 213, 2691–2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28).Mouchess M.L., Arpaia N., Souza G., Barbalat R., Ewald S.E., Lau L., et al. (2011) Transmembrane mutations in Toll-like receptor 9 bypass the requirement for ectodomain proteolysis and induce fatal inflammation. Immunity 35, 721–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29).Getahun A., Beavers N.A., Larson S.R., Shlomchik M.J., Cambier J.C. (2016) Continuous inhibitory signaling by both SHP-1 and SHIP-1 pathways is required to maintain unresponsiveness of anergic B cells. J. Exp. Med. 213, 751–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30).Pao L.I., Lam K.P., Henderson J.M., Kutok J.L., Alimzhanov M., Nitschke L., et al. (2007) B cell-specific deletion of protein-tyrosine phosphatase Shp1 promotes B-1a cell development and causes systemic autoimmunity. Immunity 27, 35–48. [DOI] [PubMed] [Google Scholar]
- 31).Baumgarth N. (2011) The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nat. Rev. Immunol. 11, 34–46. [DOI] [PubMed] [Google Scholar]
- 32).Duan B., Morel L. (2006) Role of B-1a cells in autoimmunity. Autoimmun. Rev. 5, 403–408. [DOI] [PubMed] [Google Scholar]
- 33).Shlomchik M.J. (2008) Sites and stages of autoreactive B cell activation and regulation. Immunity 28, 18–28. [DOI] [PubMed] [Google Scholar]
- 34).Erikson J., Radic M.Z., Camper S.A., Hardy R.R., Carmack C., Weigert M. (1991) Expression of anti-DNA immunoglobulin transgenes in non-autoimmune mice. Nature 349, 331–334. [DOI] [PubMed] [Google Scholar]
- 35).Gay D., Saunders T., Camper S., Weigert M. (1993) Receptor editing: an approach by autoreactive B cells to escape tolerance. J. Exp. Med. 177, 999–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36).Li H., Jiang Y., Prak E.L., Radic M., Weigert M. (2001) Editors and editing of anti-DNA receptors. Immunity 15, 947–957. [DOI] [PubMed] [Google Scholar]
- 37).Kishi Y., Higuchi T., Phoon S., Sakamaki Y., Kamiya K., Riemekasten G., et al. (2012) Apoptotic marginal zone deletion of anti-Sm/ribonucleoprotein B cells. Proc. Natl. Acad. Sci. U.S.A. 109, 7811–7816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38).Pillai S., Cariappa A. (2009) The follicular versus marginal zone B lymphocyte cell fate decision. Nat. Rev. Immunol. 9, 767–777. [DOI] [PubMed] [Google Scholar]
- 39).Higuchi T., Aiba Y., Nomura T., Matsuda J., Mochida K., Suzuki M., et al. (2002) Cutting Edge: Ectopic expression of CD40 ligand on B cells induces lupus-like autoimmune disease. J. Immunol. 168, 9–12. [DOI] [PubMed] [Google Scholar]
- 40).Cambier J.C., Gauld S.B., Merrell K.T., Vilen B.J. (2007) B-cell anergy: from transgenic models to naturally occurring anergic B cells? Nat. Rev. Immunol. 7, 633–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41).Aslam M., Kishi Y., Tsubata T. (2013) Excess CD40L does not rescue anti-DNA B cells from clonal anergy. F1000Res. 2, 218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42).Liu Y.J., Barthelemy C., de Bouteiller O., Arpin C., Durand I., Banchereau J. (1995) Memory B cells from human tonsils colonize mucosal epithelium and directly present antigen to T cells by rapid up-regulation of B7-1 and B7-2. Immunity 2, 239–248. [DOI] [PubMed] [Google Scholar]
- 43).Smith K.G., Nossal G.J., Tarlinton D.M. (1995) FAS is highly expressed in the germinal center but is not required for regulation of the B-cell response to antigen. Proc. Natl. Acad. Sci. U.S.A. 92, 11628–11632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44).Hao Z., Duncan G.S., Seagal J., Su Y.W., Hong C., Haight J., et al. (2008) Fas receptor expression in germinal-center B cells is essential for T and B lymphocyte homeostasis. Immunity 29, 615–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45).Crow M.K. (2014) Type I interferon in the pathogenesis of lupus. J. Immunol. 192, 5459–5468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46).Tsubata T. (2017) B cell tolerance and autoimmunity. F1000Res. (F1000 Faculty Rev.) in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47).Bennett L., Palucka A.K., Arce E., Cantrell V., Borvak J., Banchereau J., et al. (2003) Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J. Exp. Med. 197, 711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48).Baechler E.C., Batliwalla F.M., Karypis G., Gaffney P.M., Ortmann W.A., Espe K.J., et al. (2003) Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc. Natl. Acad. Sci. U.S.A. 100, 2610–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]